Very simple question that I am overthinking... But how many degrees of freedom does the air have? Assuming let's say the air is confined in a rigid box.
3 Answers
Since Air consists mostly of diatomic molecules (N_2 and O_2), thus it is also considered diatomic. So, for diatomic molecules maximum degree of freedom is 6. But a room temperature they exhibit only 5, i.e. 3 translational and 2 rotational.
- 19
This answer is to address the comment by @El borito
Excuse me, why the degrees of freedom for diatomic molecules are 5 in a room temperature?
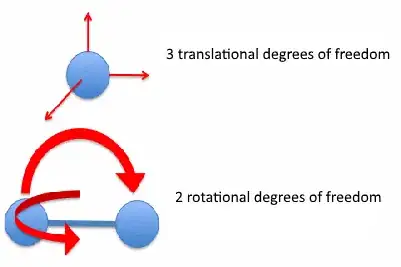
The two rotational degrees of freedom arise from the fact that the dipole can rotate in two different planes.
The three translational degrees of freedom are due to motion in the $x$, $y$ and $z$ directions (if working in cartesian coordinates)
[Remark: This image is the same as an answer given to this question which explains in more detail so should be consulted]
- 2,441
- 3
- 35
- 64
for this i think so you must first calculate $C_v$ mixture for air {i.e.of all the gases present in air $C_v=f/2+1$ where $f=$degree of freedom
- 2,441
- 3
- 35
- 64