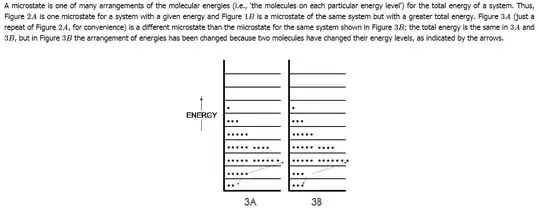
- Why various molecules in an ideal gas at a particular temperature can have only quantized energies? Why can't they have the energies distributed in a continuous fashion?
Following is an image taken from the mentioned reference, where it shows that various molecules can take only certain energies and not the energies in between those levels. What is the reason behind such quantization of energy in gas molecules?
The same idea Prof. Peter Atkins also talks about in his book "Very short introduction to Thermodynamics", but in the book, he did not explain the reason behind it.
How do we know that such energy levels are there?
What do we lose if we assume the distribution of energy to be continuous?