Can I use a normal metal antenna to emit visible light?
3 Answers
with your question you tackle one of the most rapidly evolving fields at the moment: Nanophotonics. Let me explain in the following why this is indeed the case.
Dipole antennas
It was Heinrich Hertz, student of Kirchhoff and Helmholtz who tried to verify Maxwells equations by generating electromagnetic radiation building a dipole antenna out of a somehow transformed RLC-circuit:
This is the physical ground on which we have to investigate your question to get an idea if optical antennas are possible and if you could use some of your ones to create red light. So let's look at the characteristic value of such an antenna: its length.
Length Scales
Roughly speaking, to be able to generate radiation with the mentioned form of antennas, one needs to impose a standing wave on the antenna. Assuming that the reflection at the ends of the antenna is somehow perfect, we find that an antenna will have a length of approximately $$L \approx \frac\lambda2\,.$$
If you look at the electromagnetic spectrum,
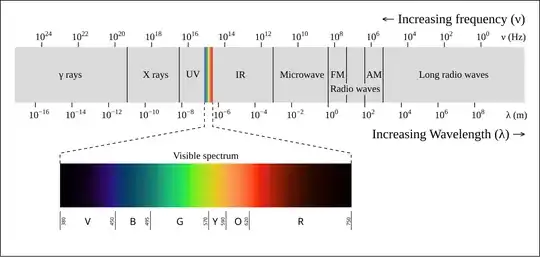
red light has a wavelength of about 600 to 700nm, your antenna will have $$L\approx 300nm$$ which is incredibly small. This is the reason, antennas in optical frequencies are subject to current research, see e.g. Resonant Optical Antennas by Mühlschlegel et al.
So, from this point of view it seems very unlikely that a Layman will be able to produce an antenna that can emit light at optical frequencies.
Sincerely
- 4,341
Yes, if you heat it :) But assuming you want to use it in the usual way the answer is no.
The color of light depends on its wavelength (assuming it's monochromatic; otherwise it will be some mixture of its components). Normal antennas have length scale on the order of centimeters to meters which is somewhat longer than microwave radiation which is still very much outside the visible spectrum.
To get visible light, you need wavelengths on the order of 100 nm (and specifically for red light around 600 nm). Where do you get such short lengths? Well, light carries energy inversely proportional to its wavelength. It turns out that these energies corresponding to visible light are often present in the absorption/emission energies of usual atoms (which is ultimately why some objects look red). Of course, this argument should in fact run backwards: the fact that our eye responds to visible light is because that's the kind of radiation to be found around us. But I digress.
And as alluded to in the beginning, you can also get red light by heating stuff. This is because at certain temperature the thermal energy will be just right to produce (mostly) red light.
In any case, to produce red light (in particular coherent, as in lasers) you need to use the above observation about absorption and emission and base your device on semiconductors, crystals, etc.
- 23,981
Don't forget that the mechanism whereby ordinary atoms emit visible light is not really any different from what classical antennas do, as I show in this blogpost. An excited atom is in a superposition of two quantum states, and the superposition of states gives you an oscillating charge distribution. I use the Larmor Formula to calculate the radiation from that oscillating charge distribution and I get the same power output as you get from using Fermi's Golden Rule to calculate the probability of a quantum leap between the two states.
- 4,289