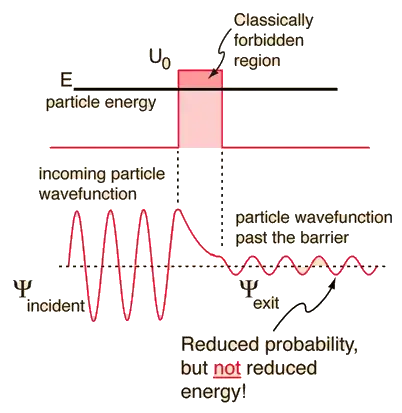
According to my understanding quantum mechanics, the probability of any particular particle in the universe being at any specific location in the universe is very small but never actually becomes zero. Thus, a little bit of all of us is everywhere.
Let's assume I suddenly quantum tunneled from sea level to the top of Mount Everest. That jump represents a net increase in energy/mass for Earth, which would violate the first law of thermodynamics. My calculations indicate it would have increased the mass of Earth by around 0.0858657µg, unless it is somehow offset some other phenomenon.
It seems implausible to me that energy conservation for a closed system could actually be violated, so how would that net increase in energy/mass be accounted for?
Or does the fact that such a scenario by definition represents such a significant decrease in the entropy of the system mean that an increase in mass should be the expected result?