Water is intrinsically blue because of molecular vibrations. The topic is covered here: Only sea water appears blue in color, why this is not happening in river water?
And here, which I post because it also includes why heavy water is not blue.
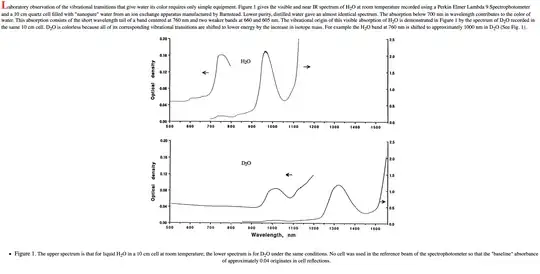 http://www.dartmouth.edu/~etrnsfer/water.htm
http://www.dartmouth.edu/~etrnsfer/water.htm
I have read that ice is less blue and more turquoise, from which I take that on freezing the absorption spectrum is shifted slightly towards longer wavelengths and lets thru more green - like deuterium but to a lesser degree.
The questions:
1: would heating (liquid) water shift the absorption spectrum towards shorter wavelengths and make water appear more violet? As it gets hotter and hotter (but pressure compels it to stay liquid) would the apparent color move more and more from blue to violet?
2: Would light water made with the isotope O15 appear more violet for the same reason heavy water has no color?