Why can the energy of a photon be directly absorbed by an optical phonon but not by an acoustic phonon?
2 Answers
You need to consider both momentum and energy conservation. The speed of sound for most materials is so low that you can't conserve both quantities for a single photon to acoustic phonon absorption.
Think about the energy of a photon that is 100 meV (comparable to optical phonons). Then it has an momentum of roughly 0.5 um$^{-1}$. At this momentum, the highest acoustic phonon for any common material (e.g. diamond) is about 0.004 meV, so it cannot absorb the photon.
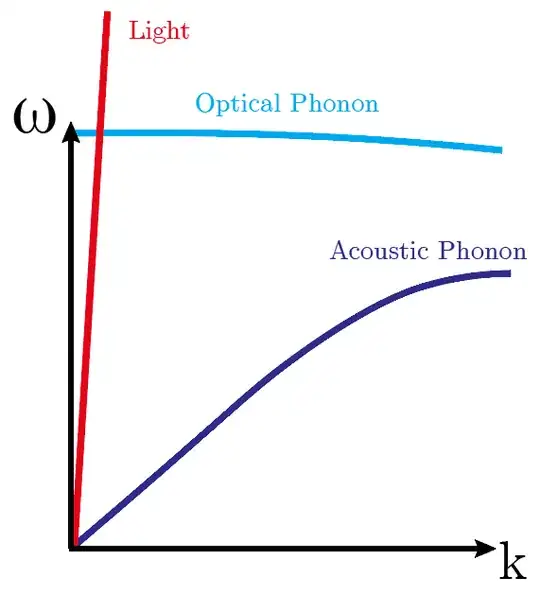
In pictures, look at the following figure showing the dispersion relations for light, optical phonons, and acoustic phonons $\omega=f(k)$. Which curve does the light line (red) intersect?
The light line can only intersect the optical phonon branch, never the acoustic one (except at zero energy, which is irrelevant). This is due to the velocity mismatch between the modes, which essentially means that the speed of sound is always much smaller than the speed of light.
However, using this same diagram, you can imagine multi-photon processes where you can excite acoustic phonons. There, you usually have a standing want of photons whose momentum difference matches the acoustic phonon. This is the principle behind Brillouin scattering, for example. These processes are generally much much weaker than direct transitions, but are still visible in certain circumstances.
- 8,314
Phonons have different modes of excitations. One kind of excitation is called the acoustic phonon. This kind of phonon has a dispersion relation like $\omega= ck$ for small k and where c is the speed of sound.
The optical phonon, however has a different dispersion relation, where $\omega\to\text{(non-zero constant)}$ as $k\to0$. In an optical phonon, neighbouring atoms move in opposite directions. In an ionic crystal, since neighbouring atoms have opposite charge, this is an oscillating dipole that radiation can interact with. It turns out that the large wavelength (small k i.e. wavenumber) optical phonons have energies similar to infrared radiation, so they can absorb light. I.e. light can excite optical phonons.
The acoustic phonons have very small energy/frequency (relative to light) for large wavelengths (small wave number) since $\omega\propto k$ for small k, so do not have strong coupling so to light. Also, they (generically) do not set up an oscillating dipole like the optical phonons.
See also Fig 22.10 of Ashcroft & Mermin.
- 526
- 4
- 19