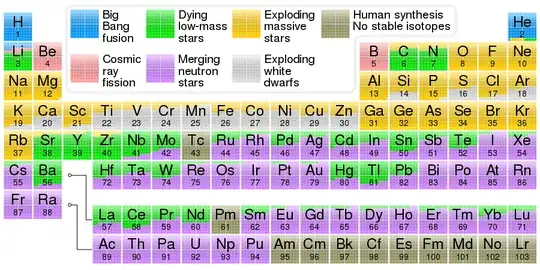
We all know where iron comes from. However, as I am reading up on supernovas, I started to wonder why there is as much iron as there is in the universe.
Neither brown dwarfs nor white dwarfs deposit iron.
Type I supernovas leave no remnant so I can see where there would be iron released.
Type II supernovas leave either a neutron star or a black hole. As I understand it, the iron ash core collapses and the shock wave blows the rest of the star apart. Therefore no iron is released. (I know some would be made in the explosion along with all of the elements up to uranium. But would that account for all of the iron in the universe?)
Hypernovas will deposit iron, but they seem to be really rare.
Do Type I supernovas happen so frequently that iron is this common? Or am I missing something?