The mass of free neutron is around 0.1% more than the mass of proton. When it is bound inside the nucleus, neutron mass is less compared to its mass in free state. The question is how much mass it loses? How is the mass in the bound state compared to mass of proton? I am assuming now the difference is less than 0.1% but how much less?
2 Answers
In most nuclei the binding energy is around 7 - 9 MeV per nucleon.
Picture from Wikipedia:Nuclear binding energy:
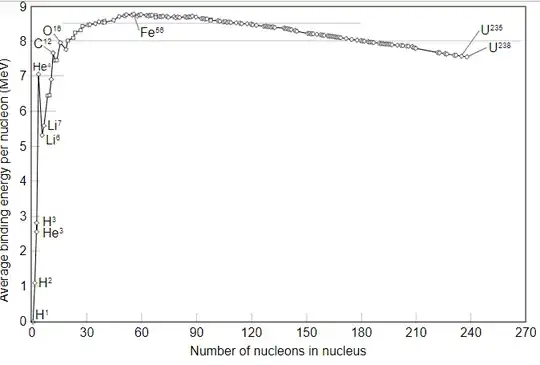
Comparing this to the rest mass of free protons (938 MeV) and neutrons (940 MeV), this means the total mass of a nucleus is around 0.75 - 0.95 % lower than the sum of the free nucleons.
However, it is hard to say anything about the masses of individual protons/neutrons in the nucleus, because you can only determine the total mass of the nucleus, but not the mass of a single proton/neutron inside the nucleus.
- 42,352
It was already commented in this sense, I just try to turn the comment into the answer. The problem is already in the statement
When it is bound inside the nucleus, neutron mass is less compared to its mass in free state.
It is not possible to view the bound state as a set of independent particles that have lost their weight.
There is number of models (perspectives) that the system may be regarded. From a soup of quarks, soup of appearing and disappearing mesons, a droplet, rotating/vibrating elipsoid up to the system of interacting particles in a mean potential (however not regarded as massive particles in gravitational field, but rather things occupying the quantum states available).
Basically, the interactions of the particles lead to the lower weight (higher binding) of the system.
- 1,994