Methane’s absorption bands are centered at 3.2 and 7.2 microns -- far off the peak of the Planck spectrum for a 290 K blackbody near 16 microns. Moreover, its absorption bands overlap with the water absorption region. So why is methane considered a stronger greenhouse gas than carbon dioxide, which has an absorption band at 15 microns?
7 Answers
I'm just guessing that it's due to temperature- and pressure-broadening of the line shapes for CH4 versus CO2. You have to integrate the radiative transfer equation over frequencies as well as over an atmospheric column. So the line shapes, as well as the lines, also play a crucial role determining the overall effect. They're not just $\delta$-function-like. But I'm not immediately googling the answer I'm suggesting here. Anybody finding a cite?
E d i t
--------
Re @BertBarrois's comment below, let me suggest a totally different mechanism that might contribute to Bert's "methane problem".
CO2 is well-mixed in the atmosphere, whereas I don't know whether or not methane is. For our purposes here, let's suppose that (like ozone) methane isn't well-mixed. Then we can suggest the following.
All absorbed radiation is eventually reradiated (though possibly in several steps, i.e., several photons, at lower frequency). Now, the outgoing thermal radiation we're discussing is mostly coming from the Earth's surface (although a non-negligible part comes from atmospheric reradiation). And that surface radiation is non-isotropic, i.e., it's all from the ground going up. Greenhouse blanketing arises because the reradiated radiation >>is<< emitted isotropically, meaning that some of the radiation originally on its way up-and-out, gets sent back down to the surface. And then the whole process repeats, so to speak, with the net result that the equilibrium temperature gets warmer.
And part of that equilibrium temperature dependence is the vertical atmospheric layer where the reradiation occurs; closer to the ground means warmer. So that may contribute to the lesser effect of well-mixed CO2 versus (what we're supposing is) CH4 absorber amounts closer to the ground.
My own answer …
The IPCC report rates the global warming potential of greenhouse gases in terms of the incremental effect of each additional kilogram on IR transmission. The context includes all important greenhouse gases at baseline concentrations.
Even if CO2 and CH4 were examined out-of-context, it is difficult to estimate their relative oscillator strength from first principles, and collision rates are a further wild card. Per QM, the power radiated by a vibrating diatomic molecule with fractional effective charges $\pm Q$ at the ends should scale as ${{Q}^{2}}{{\omega }^{3}}/m$, where $m$ denotes reduced mass and $\omega $ vibrational frequency. The latter parameters are known, but only a brave chemist would dare to guess at the fractional effective charges based on the polarity of covalent bonds.
The IPCC report’s fundamental metric is radiative forcing, which quantifies the incremental effect of additional greenhouse gases on IR transmission, not from the ~288-K surface through the entire atmosphere, but from the ~220-K tropopause through the stratosphere. Since the CO2 absorption band centered at 15 microns is almost completely blocked (“self-saturated”) under baseline assumptions, extra CO2 merely widens the shoulders of the band. The CH4 band centered at 7.2 microns is almost completely blocked by water vapor within the troposphere, but the stratosphere is very dry, so CH4 has ample headroom to make trouble there. Wikipedia has nice figures of the stratospheric transmission spectra.
The opacity of the lower stratosphere in blocked bands governs the temperature gradient needed to drive radiative transport, which in turn governs the altitude of the tropopause, because convection gives way to radiative transport wherever the adiabatic gradient exceeds the radiative gradient. Each extra kilometer of altitude could raise the surface temperature as much as 6.5 degC, according to the effective lapse rate of the Standard Atmosphere, and the theoretical adiabatic lapse rate in dry air is even higher, 9.8 degC/km.
CH4 may never be as important as CO2 because it is much less abundant -- currently about 2 vs 400 ppm -- but gram-for-gram, it makes a greater marginal contribution to radiative forcing.
- 3,147
Basically it’s because methane has a more complex physical structure than carbon dioxide. It has five atoms and four bonds compared to carbon dioxide which has three atoms and two bonds.
This means that it can vibrate in more ways than carbon dioxide and hence it absorbs thermal radiation more strongly.
This is why it’s GWP (Global Warming Potential) is so high (at 21 x that of carbon dioxide).
- 14,713
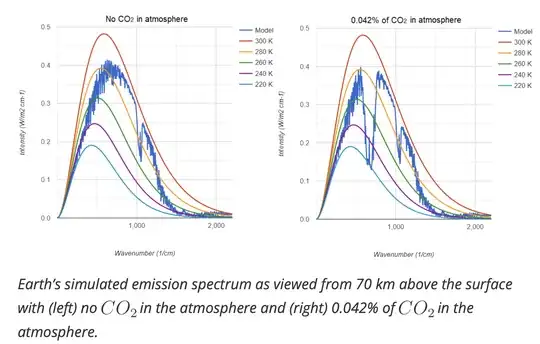
The answers here are all highly speculative and are all also missing a crucial point about the Greenhouse Effect: when considering the greenhouse effect, we are more concerned with relatively low-frequency absorption bands than high-frequency absorption bands. To see why, compare the following two blackbody emission spectra taken from this post that show the simulated emission spectrum of the Earth with and without the 0.04% CO$_2$ contribution in the atmosphere.
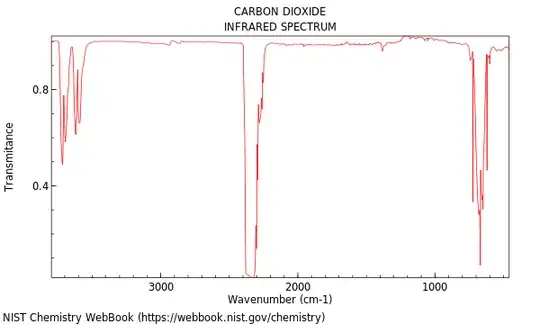
This is a pretty striking difference! The difference comes from the strong absorption band in the CO$_2$ spectrum around 600 cm$^{-1}$, as shown in its IR spectrum,
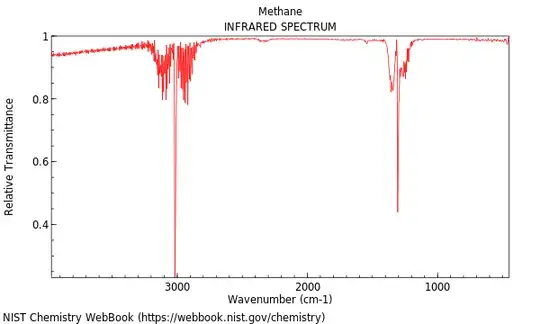
Also notice that, with or without greenhouse gases, there is little emission corresponding to higher-frequency radiation since our atmosphere already has a significant amount of gases that absorb in those regions. But methane also has a strong absorption band in its IR spectrum relatively near the region of maximal emission from Earth, as shown in the following,
Worse yet, this band does not directly overlap with the most intense part of either the bands for H$_2$O (shown below) or CO$_2$, which means that it can also contribute to the Greenhouse Effect. It should be emphasized that methane is an extremely trace gas, however, and that given the growing preponderance of CO$_2$ we should really be more concerned about its obvious effects on our climate.
- 3,857
I have not been able to find a good explanation for why methane is a strong greenhouse gas despite its absorption properties (stated in the question), but I think I can contribute some relavant information.
I think the main fact to understand is that the greenhouse effect is more complicated than the common popular science explanations.
The simplification
The popular explanation usually is something similar to this diagram:

From Wikipedia: https://en.wikipedia.org/w/index.php?title=Greenhouse_effect&oldid=1148217994
This is just a simplification. (In my opinion, too much a simplification to be justified.)
A somewhat more accurate illustration is this:
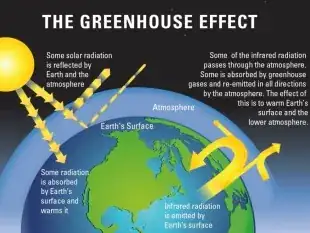
From Royal Society: https://royalsociety.org/topics-policy/projects/climate-change-evidence-causes/basics-of-climate-change/
Or this:

From UCAR: https://scied.ucar.edu/learning-zone/how-climate-works/greenhouse-effect
This illustrates that most of the thermal radiation from the surface escape into space. In stead almost all of it is absorbed by the atmosphere, which is heated, and radiates its heat to the rest of the atmosphere. It is in the topmost layer of the atmosphere that radiats the heat into space.
This is explained well in this video by Sabine Hossenfelder: https://www.youtube.com/watch?v=oqu5DjzOBF8
The explanation
I have only found a few titbits.
- The more complex explanation of the greenhouse effect makes it clear that the absorption band of a gas is only a small part of its effect.
- The molecular structure of methane makes it able to absorb far more heat than carbon dioxide, the 4 hydrogen bindings all can "jiggle". (I have not understood how this works and why it is relevant.)
- When methane breaks down in high altitudes it forms water vapour, which creates stratospheric clouds, which prevent head from escaping into space.
- When methane breaks down in high altitudes it forms ozone, which is a greenhouse effect in itself.
Assorted popular science sources:
- 103
I have done a complete simulation that shows that methane is trivial compared to H2O that does block the methane bands. This was done using the HITRAN database and physical properties of atmosphere. So I agree with above that the Oxford/EPA/IPCC judgement of being 28 times more powerful than CO2 is erroneous, other than the fact that CO2 bands are mostly self-saturated. Wikipedia is a little misleading on this account by stating that CO2 is non-linear. There can be stiffening and softening non-linarities, they should just state this is the law of diminishing returns for CO2 due to self-saturation (I guess you would class this as a stiffening non-linearity). On a per mole basis you can see that CO2 and H2O are stronger absorbers than methane just by consulting the HITRAN database. In the original study in 1995 Oxford had to do a lot of fancy footwork to flip this to the opposite conclusion. One part of this was there assumption that ozone in the atmosphere would reduce and increase the lifetime of methane in atmosphere by reducing the number of oxidization events. In fact the ozone has increased since then due to restriction in CFCs released.
- 1
- 1
I'm quite surprised no once has cited the March 2023 Wijngaarden and Happer paper "The Role of Greenhouse Gases in Energy Transfer in the Earth’s Atmosphere". The very short story is that in fact H2O overpowers the CH4 absorbtion bands, therefore CH4 is basically irrelevant when it comes to the greenhouse effect. The short story is here (a summary of the paper) https://andymaypetrophysicist.com/2021/09/20/the-greenhouse-effect-a-summary-of-wijngaarden-and-happer/ and the complete paper is here: https://co2coalition.org/wp-content/uploads/2023/11/The-Role-of-Greenhouse-Gases-in-Energy-Transfer-in-the-Earths-Atmosphere.pdf
I have seen no reasonable rebuttal to the Happer paper - and its atmospheric modeling is shown to be correct by measurements/observations.
- 1