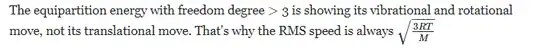 in my notes from class I have that
in my notes from class I have that
Vrms= sqrt(3kT/m) for a pt molecule Vrms= sqrt(5kT/m) for a diatomic molecule and that Vrms= sqrt(6kT/m) for a triatomic-> higher order molecule
but RMS Speed of Gas Molecule for Polyatomic Molecules
says that Vrms=sqrt(3kT/m) always and I don't understand why that is.
Thanks,
I know another post has about the same question- but wasn't able to comment or add a related question due to my 0 reputation.
Don't understand where Vrms formula comes from. Is it solely dependent on translation motion. And that's why for all molecules you can use the same exact Vrms formula.