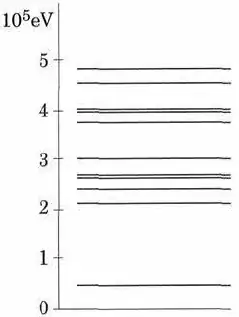
In my textbook I was asked to compute the wavelength of a photon emitted as the nucleus of a Sodium atom pass from the first excited level to the base level. I got the formula itself right, but inserted the wrong difference in energy between the two levels when I checked the answer key. The number I used was 0.4 x 10^5 eV, based on the attached graph, assuming that the base level is at point 0 and the first excited level is the first line above what I assumed to be the base level (around 0.4 x 10^5 eV). The answer key uses the amount of 1.5 x 10^5 eV, based on the same graph. Was I wrong in my way of reading the graph, in the sense that the base level is not at 0, but rather the first line (around 0.4 x 10^5 eV), with the first excited level being at 2.2 x 10^5?
I need to know so that I am able to read these graphs correctly in the future.
Thank you!