Tree-like structure in chocolate
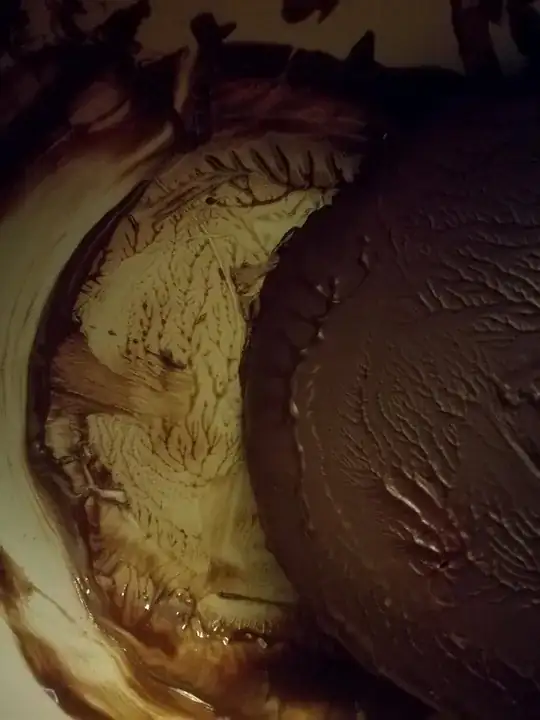
Today I let some melted chocolate solidify in a smooth bowl in my fridge. When it had settled, I gently heated the outside of the bowl with warm water to unstick the chocolate. It caught my attention that the remaining chocolate (both in the bowl and on the larger piece I removed) formed a beautiful, fractal tree-structure of sorts. I noticed that the various branches in the tree do not seem to intersect each other.
Next it occured to me that I had seen a similar thing happen to soft butter on a cold knife. I suspect the effect emerges because the fatty compounds in chocolate/butter try to minimize their surface tension (like water forming droplets), but why is the minimum-energy-configuration a non-self-intersecting, fractal branch structure, and not simply droplets? Could it be some tradeoff between surface tension, viscosity and density?
EDIT
User, Bert Hickman, suggested the effect might be an example of a "Hele-Shaw cell". This apparatus is used to demonstrate "Hele-Shaw flow" of a liquid between two plates:
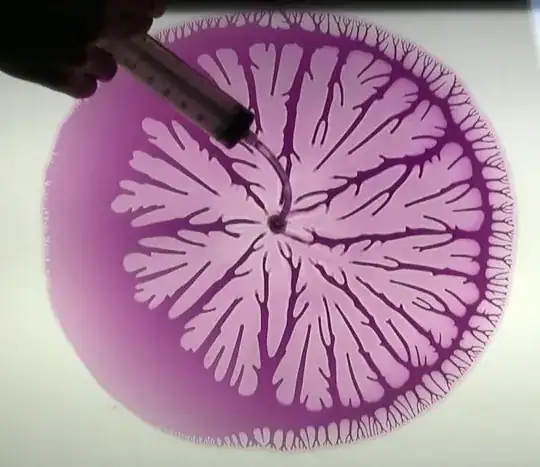
This explanation makes sense considering what I did to the chocolate. When re-heating it the chocolate between the bowl and the upper layer is liquified. Once I remove the upper layer air rushes in and creates the peculiar flow. I found this nice video demonstrating the effect with water and oil.