Is there a specific pressure that is needed to boil water at room temperature? If there is, what is it? Why does water boil at a low pressure at all?
Asked
Active
Viewed 1.6k times
2 Answers
7
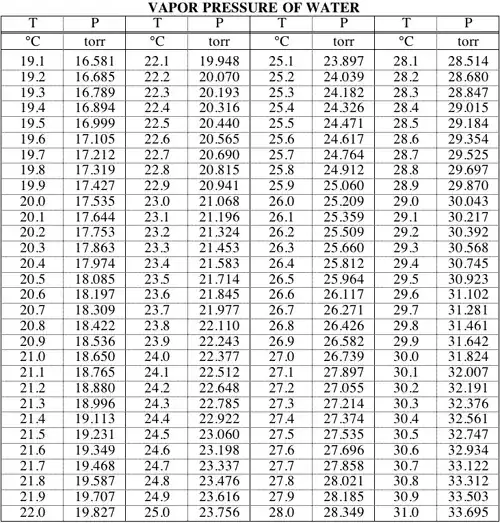
Since boiling, by definition, occurs when a liquid's vapor pressure reaches ambient pressure, your question is identical to asking what the vapor pressure of water is at room temperature. Here's an example of an online table:
At 23°C, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure.
Chemomechanics
- 30,163
2
What causes water to boil is not only the ambient temperature but rather the pressure acting upon the water surface. Example, at sea level where atm. Pressure is 14.7 psi, water will start to boil at 212 degrees F. However. At a higher elevation, say in Denver, Co. That is 5,250 feet above sea level, the pressure acting on the water surface is lower and thus, water will boil at a lower temperature.
Gerard De Santis
- 126