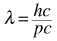Does the wavelength of photon affect on it's size as when the wavelength is big the photon has big size and it small it has small size??
No, photons are point particles in the standard model of particle physics, a quantum mechanical model. They have zero mass and energy equal to $h*ν$, where ν is the frequency of the classical wave that will be built up from a large number of the same energy photons. Here is a record of single photons , they have the footprint of a point in the detecting plane.
What the wavelength does is define the probability of detecting the photon, so accumulated measurements under same boundary conditions are needed to see the wave nature of a photon.
In the electronmicroscope the electron should poses a small wavelength ,does the wavelength of electron affect also on its size ?
Again, for quantum entities as the elementary particles, no size exists. For an electron it is the de broglie wavelength,
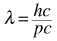
dependent on the electron momentum, which will control the probability of measuring the electron at an (x,y,z) at time t. It is the probability that may show wave effects, depending on the boundary conditions.
and I know when the electron accelerate its mass increase due to its velocity
This is a misconception, the mass you are describing is the relativistic mass and is not to be used in the de Broglie formula. The momentum there is with the invariant mass which does not change with energy as the name says.
but it's wavelength is small how it can penetrate the bodies and form a more qualified image than photon if it has more mass and thusit will have a big size
No, the size of the electron is always a point, what changes with momentum is the probability of interacting with the atoms and molecules under observation. The higher the energy the smaller the wavelength and accumulation of electrons will show the probability of scattering from the lattices under examination, which will depend on the wavelength.
is there any relation between the wavelength and the size of both electron and photon ?
No, see above.