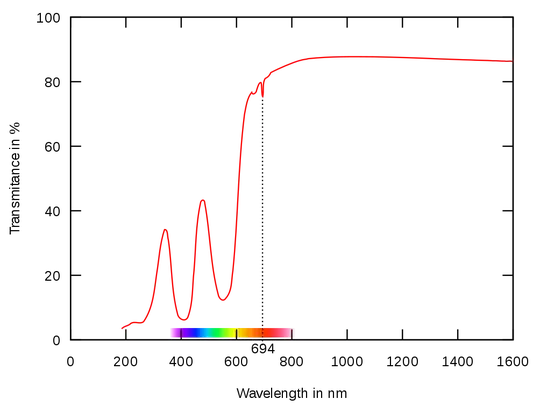
You might have seen the absorption spectrum of ruby: it has distinct absorption peaks near 400nm and 550nm. Looking at its absorption graph, one could expect some fluorescence when excited from a ~450nm source - not as good as from a 400nm source, but absorption is still significant.
I've compared the fluorescence of a ruby rod when illuminated by 405 and 450-460nm LEDs. It appeared that fluorescence from a blue LED is extremely weak, many dozens of times weaker than from a 405nm LED. But from the absorption graph, the difference is not that great.
Does anyone have insight on why there is absorption, but almost no fluorescence of ruby when illuminated by a 450-460nm source? The same for a 532nm laser source (i.e. narrow band this time, but slightly higher energy than needed) - there is absorption, but almost no fluorescence.
Has someone seen fluorescence activation spectra in the literature? On the ruby state diagram I see that the main "up" transitions are activated by 420nm and 550nm photons, but it is not clear how wide the peaks are for crystalline (= almost perfect) synthetic ruby.
The goal is to find current / future semiconductor sources which would allow DPSS Ruby laser.