I just don't understand one thing, if photons carry energy, shouldn't
the electron be forced to higher excited states?
This typically does not happen because the photon usually considered when discussing spontaneous emission is not on resonance with any of the higher excited states.
But you are correct that what you describe can happen.
Consider the following diagram:
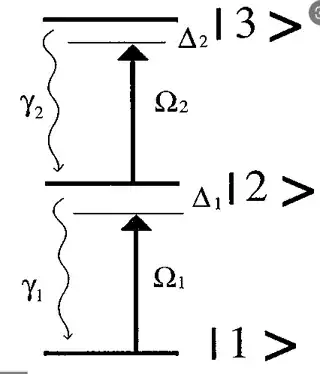
A photon can make the state transition from $|1\rangle \rightarrow |2\rangle$, if the photon is the right energy and the atom is in state $|1\rangle $. But they key is that the photon needs to match the energy level difference between 2 and 1. Likewise, the same thing can happen if the atom is in $|2\rangle$ initially and the photon has an energy level difference equal to the energy level difference of the excited states 2 and 3.
So, we can get what you described if we sent a photon with energy $\omega = \omega_3 - \omega_2$ when the state is $|2\rangle$, then it will transition to $|3\rangle$.
But what if we sent a photon with energy $\omega = \omega_2 - \omega_1$ when the state is $|2\rangle$? Now it can't get excited again to the excited state $|3\rangle$ because its too far away in frequency. This is the case that causes stimulated emission. When light is sent on-resonance (same energy) with a transition, it generally causes the atom to flip-flop between excited states. So light will cause the atom to transition from $|1\rangle \rightarrow |2\rangle \rightarrow |1\rangle \rightarrow |2\rangle \rightarrow ...$. When you consider just a single photon, then essentially only a single step of this process can happen. In $|1\rangle \rightarrow |2\rangle$ a photon is absorbed, in $|2\rangle \rightarrow |1\rangle$ an extra photon is created.
