I know that black absorbs light and converts it into heat which makes it a good emitter of radiant heat while white reflects it. Let's say if I place 2 cups, 1 black and 1 white, same material, in a dark room, which cools faster? Why is black a better emitter? Is it because it converts light to heat?
1 Answers
The existence of equilibrium demands that emissivity is equal to absorptivity.
As long as a body is, say, emitting more energy than absorbing, its temperature will be decreasing: therefore, as thermal equilibrium imply all parts of the system share the same temperature, for equilibrium to be reachable, the body's emissivity must equal its absorptivity. See the Kirchhoff's law of thermal radiation.
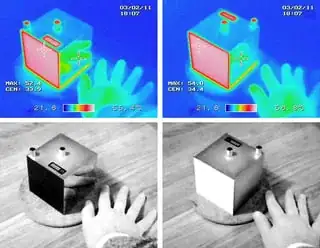
Another point to consider is that being black or white are object characteristics with respect to visible frequencies of light, while at room temperature most of the emission/absorption happens at lower frequencies (infrared). For example, the infrared pictures of the aluminum box below make it evident that the emissivities of its white and black surfaces are very similar (as explained in its manual).
Source: Wikipedia
Answer: Now, if the cups are "black" and "white" at infrared, than the black cup will cool faster, since it's emitting energy through radiation at a higher rate than the white one (and absorbing less then emitting, since the environment is cooler).
- 13,064