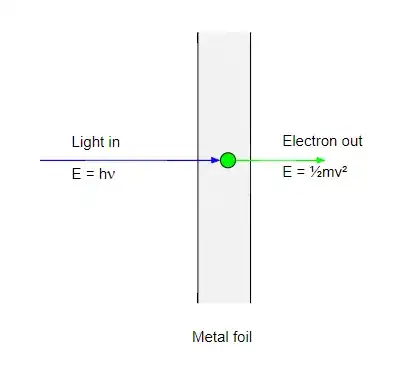
If you leave light shining onto that metal, for long enough, does the energy of the individual photons accumulate, on the electrons, so eventually they will ionize, or does this not happen? What am I missing?
Here is what happens :
Electrons ejected from a sodium metal surface were measured as an electric current. Finding the opposing voltage it took to stop all the electrons gave a measure of the maximum kinetic energy of the electrons in electron volts.
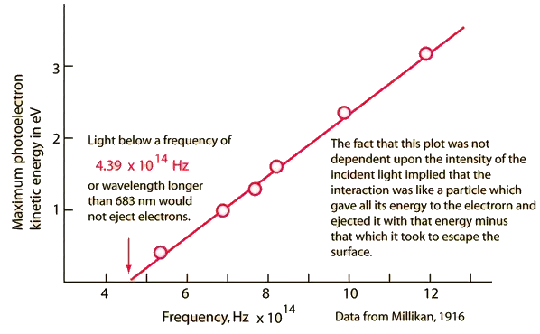
The minimum energy required to eject an electron from the surface is called the photoelectric work function. The threshold for this element corresponds to a wavelength of 683 nm. Using this wavelength in the Planck relationship gives a photon energy of 1.82 eV.
If you keep hitting with electromagnetic radiation an opaque metalic material below the critical frequency, the temperature of the material will go up because of atomic and molecular and lattice absorption of the energy of a portion of the impinging photons . This energy will raise the temperature of the lattice, and thuswill also raise the energy of the conduction electrons,
The conduction electrons can, therefore, be treated as an ideal gas. However, the concentration of such electrons in a metal far exceeds the concentration of particles in a conventional gas. It is, therefore, not surprising that conduction electrons cannot normally be analyzed using classical statistics: in fact, they are subject to Fermi-Dirac statistics (since electrons are fermions).
....
It is clear, from the above equation, that the Fermi energy $\mu$ is generally a function of the temperature $T$.
The work function of the photoelectric effect depends on this energy
As this paper shows, the work function will change with temperature
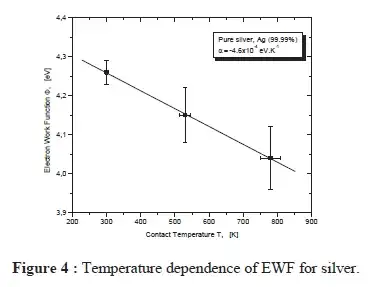
The results for pure silver metal show an evolution of the work function with temperature; a decrease of this parameter of about 0.2 0.3 eV is observed when the cathode temperature reaches 700 K, independently of the nature of the material. The obtained results are rather approximate. This method is not very accurate at higher temperature, because above 700 K the thermionic current dominates and the measurements of the photoelectric current are falsified by large fluctuations
In this sense , if the frequency is below the frequency of the work function by a tiny amount, the rising of the temperature from the absorbed photons will push the fermi level energies high enough so that the photoelectric effect can appear.
So there will be an effect, but it is a collective thermodynamic one, not on individual electrons as explained in John's answer, because of the 1/137 drop of the crossection (due to the coupling constant) for each photon interaction.