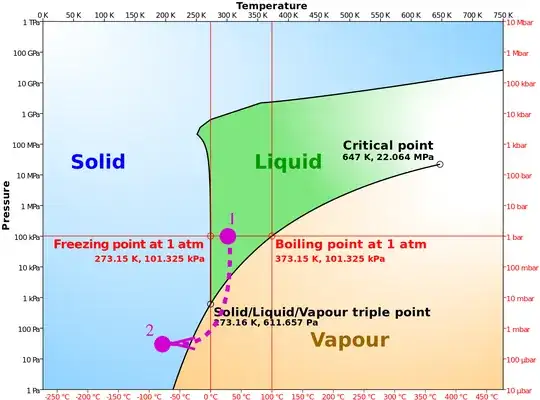
In this technique Vacuum is created in the chamber and water is placed in the chamber. As the pressure decreases so the boiling point also decreases and water start boiling and evaporation starts. leaving behind the solid ice. My question is that how ice is made. Is it like that water takes energy to boil from itself, leaving behind the solid ice?
2 Answers
water takes energy to boil from itself, leaving behind the solid ice
Yes, that's essentially what happens. If it feels strange, remember that the process of evacuation is removing energy from the chamber.
Why this is so is easy to see in the traditional example of a moving wall (or a piston) increasing the volume of an adiabatic chamber: the gas in the chamber performs work on the wall, $$ W = \int F\,\mathrm{d}x = \int pA\, \mathrm{d}x= \int p\, \mathrm{d}V > 0\,\,\text{ (since $p>0$ and $V$ increases)},$$ i.e., the moving wall forces the gas to transfer energy to the environment, and it can only do so by cooling itself. EDIT: That's an equilibrium thermodynamics description that is not very relevant here. See bellow.
What is going on, considering the concrete case of a chamber, is:
- initially there is air and some liquid water at room conditions (1);
- the chamber is then evacuated and pressure drops below the boiling point;
- the water looses energy through ebullition and evaporation, and freezes (2).
Original picture source: Cmglee, https://commons.wikimedia.org/w/index.php?curid=34865054
Some important points:
- There is a cool Youtube video demonstrating this phenomenon.
- That's an out-of-equilibrium process, so thermodynamical variables are at times undefined, and some common assumptions might be unjustified;
- The drop of pressure in the chamber is not the drop experienced by the water, due to surface tension.
- A big chamber (with respect to the amount of water) or the continuous removal of water vapor might be important for freezing to occur.
- This nice answer to the question Water in vacuum (or space) and temperature in space provides some calculations, and qualitatively corroborates the reasoning above.
- 13,064
Actual Phenomenon behind the vacuum freezing is low pressure boiling. When the pressure is lowered the boiling point of water decreases. At very low pressure the water start boiling and takes heat of vaporization form water itself. Thus, decreases the temperature of water and eventually water is converted in to ice. More or less it is similar to the phenomenon occur in the evaporator of air conditioner, where refrigerant boils at low pressure and takes this energy of boiling from the room to be conditioned thus decreasing the temperature of the conditioned room. Hope this answer is helpful for all. Thanks
edit: This phase diagram for water from here shows that by moving from atmospheric pressure at 100 kPa down to about 600 Pa, the freezing point and boiling point of water converge to the same temperature. Near this point, the boiling of the water removes enough heat to cool the rest of the water enough for it to freeze. That point at which all three phases can exist at the same time is called the triple point.