As per Coulomb's law positive charges must repel but in the the nucleus of an atom positively charged protons stay together, why is this so?
2 Answers
Short answer :
Within the range of 2 fermi $(1\space fm=10^{-15}m)$, strong nuclear attractive forces overcome the relatively weaker repulsive forces of protons.
These nuclear forces are attractive in nature and independent of the charge of the particles. Neutrons attract neutrons, neutrons attract protons and protons attract protons.
If this force was absent, stable nuclei wouldn't exist.
- 4,937
- 4
- 25
- 38
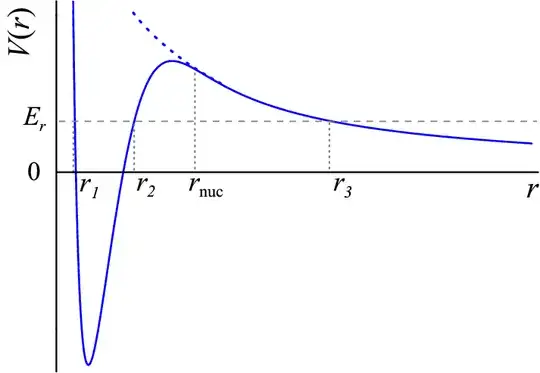
The picture bellow schematically shows the Coulomb repulsion by a dashed blue line.
The sum of Coulomb and nuclear force is in solid blue.
If you manage to get the nuclei close enough, they interact via the strong force and can overcome the Coulomb barrier.
Sometimes, in some configurations, the nuclear force is not able to create a bound system (it is energetically more favored to go away) - so you will not find bi-proton. And you also cannot find bi-neutron, despite there is not charge messing.
- 1,994