When a substance absorbs and reflects light, it shows the color of that absorbed and reflected light. But water is colorless. Does it absorb and reflect light. What is the reason for this?
4 Answers
Liquid water has very little absorption in the visible light - this is why it looks colorless.
There is a very extensive article on the absorption of electromagnetic radiation by water on Wikipedia.
A few highlights:
- there is a difference whether you are talking about solid (ice), liquid ("water") or gas (vapor). This has to do with the fact that much of the absorption is a result of molecular vibrations - and not all vibrations are possible in all states
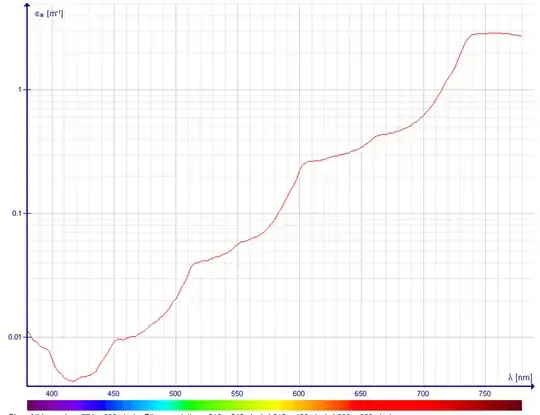
- Liquid water has very low absorption in the visible spectrum. Here is a plot of the absorption (note - this is for PURE water; contamination will greatly affect the transparency. For example, fine particles in water will preferentially scatter blue light):
Attribution: Darekk2 on Wikipedia, based on
- Pope R. M., Fry E. S. Absorption spectrum (380-700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 36, 8710-8723 (1997), eprint.
- Kou L., Labrie D., Chýlek P. Refractive indices of water and ice the 0.65- to 2.5-µm spectral range. Appl. Opt. 32, 3531-3540 (1993), eprint.
From the Wikipedia article:
The absorption was attributed to a sequence of overtone and combination bands whose intensity decreases at each step, giving rise to an absolute minimum at 418 nm, at which wavelength the attenuation coefficient is about 0.0044 m$^{−1}$, which is an attenuation length of about 227 meters. These values correspond to pure absorption without scattering effects.
- 137,480
- 119,981
Water does not absorb much light in the visible range so most visible light simply passes through. Water is, however, opaque to some other wavelengths such as microwaves.
- 4,936
Our eyes are made of watery parts. If water DID strongly absorb light of some color, it would necessarily be a color that we cannot see, for which we have no name. Water vapor and many other atmospheric gasses are natural limits on ambient light, so it is not surprising that we describe them as 'transparent' to visible light.
Water (liquid and vapor) has some color in the far-infraredIR spectrum, with absorption at 3, 6, and 12 microns. So-called 'glacier ice' can be a striking blue color (possibly because of impurities like trapped air).
- 10,853
When a substance is transparent and reflects no color, at the particle level it means that the photons on which classical light rides have only elastic interactions with the medium, i.e. the atoms and molecules, and do not raise an energy level in the optical range so that the balance of perceived colors is changed.
Take a red colored crystal , a beam of light going through it turns red. At the particle level it means that there is absorption of photons from the atoms and molecules and the lattice so that the mix of frequencies coming out is perceived as red.. The same would happen if you pour red ink in water. The extra molecules of the red ink absorb the appropriate frequencies to leave the frequencies perceived as red.
Please note that the energy of the absorbed photons raises an electron to a higher level or changes the lattice position of an atom/molecule . In de -excitation there could be cascades to lower levels with a change in frequencies, but also the angular distribution of the photons coming from the de-excitation will be spherical and coherence with the beam would be lost. This means that when images are transmitted the process is elastic in order that coherence is not lost.
- 236,935