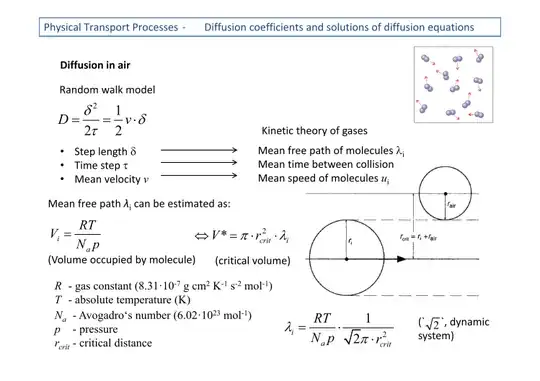
In the equation in the picture, the mean free path $\lambda$ is described as the volume occupied by a molecule, divided by the volume of the molecule times root two. I do not exactly grasp the purpose of dividing by $\sqrt{2}$, could someone explain it?
1 Answers
The calculation done above assumes that one molecule is moving, while all of the others are standing still. This is a much easier situation to handle; you basically just count up how much volume is occupied by other molecules, and how fast your one moving molecule traces out volume.
In reality, all of the other molecules are moving, so the typical relative speed between molecules will be larger. To see this, consider the one-dimensional case where the velocities of two particles, $v_x$ and $v_y$, are normally distributed with zero mean and standard deviation $v_0$. Then the relative velocity $v_x - v_y$ is normally distributed with standard deviation $$\sqrt{v_0^2 + v_0^2} = \sqrt{2} v_0$$ since variances of independent variables add. Since the typical relative speed is about $\sqrt{2} v_0$, we can imitate having all molecules moving (a 'dynamic system') by just giving a single moving molecule an effective speed $\sqrt{2}$ times as big.
- 107,105