I'm trying to understand the logic behind this famous thought experiment, my confusion is as follows:
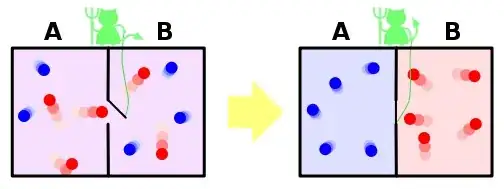
In the box on the right after the demon has finished his 'work', I understand that side A now has low entropy since the demon has only permitted fast-moving molecules into B. So the demon has rendered the side A full of $\color{blue}{\text{cold}}$, $\color{blue}{\text{slow}}$ moving molecules, which makes perfect sense and it seems correct to say the entropy of side A has decreased.
A quote I read recently says that
In an adiabatic expansion where the entropy stays constant the space available for the gas increases thus the speed of the molecules must correspondingly fall.
So my understanding of that statement is that the system is more disordered (entropy increases) if the space available for the gas increases, so to compensate the molecules must slow down to have $\bbox[yellow]{\text{less}}$ entropy (as the overall entropy change must be zero).
According to the image above, the whole system (A and B on the right box of the previous image) has a reduced entropy.
But here's the problem: Side B is now full of $\color{red}{\text{hot}}$, $\color{red}{\text{fast}}$ moving molecules.
So does this mean that the entropy of side B has increased?
Edit:
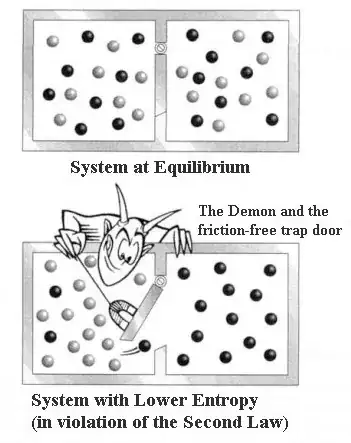
One of the comments below (which has been deleted now) mentions that the entropy of B does increase but the overall entropy of the system (A+B) decreases.
So the question remains: why does the entropy of the system decrease and not increase?
Or put another way: why is side A more 'dominant' in the overall contribution of the system's entropy?