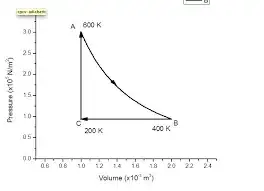
Is it true that a pV diagram of a thermodynamic cycle with a specified scale on both axes is enough to calculate its efficiency (such as the one below)? Is it the area within the shape divided by the area under the top curve?
2 Answers
If the graph itself really is the only information that you have, then you're out of luck. But in many cases you will actually have some other information, and it may be enough to let yo solve the problem. If
- You also know the internal energy as a function of pressure and volume $E = E(P,V)$.1
- You either known the functional form of the curves in diagram or are willing to settle for an approximation to the work for each stage (numeric or from a planimeter).
and
- All the stages are quasi-static
then this is tractable.
The area under each segment gives you the work done on that segment, and from the end-points you compute the total change in internal energy $\Delta E$. The heat for the stage is then2 $$ Q = \Delta E - W \;, \tag{1}$$
Add all the heat from stages with positive $Q$ together and call that $Q_H$.
Add all the heat from stages with negative $Q$ together and call the absolute value of the result $Q_C$.
The efficiency is then $$ \eta = 1 - \frac{Q_C}{Q_H} \;,$$ in the normal fashion.
1 Keep in mind that for monatomic ideal gases $E = \frac{3}{2}PV$, and that you can use $E = \frac{5}{2}PV$ for air at STP and make fairly modest error.
2 I've written equation (1) in the convention where work done on the system is positive. If you are using the convention that work done by the system is positive than it should read $$ Q = \Delta E + W \;. \tag{1a}$$
By definition, efficiency of a thermodynamic process, is the ratio of the total work done as output to the total heat supplied (and only supplied, mind it). It is very important to add only the heats that are supplied(I am specifically using plural form to emphasise on this fact) and not the net heat exchange for the process. If in the diagram, certain values(say pressure, volume etc. as in your graph) are provided with at some points, then you may surely find the efficiency. For each curve, you would need to find out whether heat is supplied or released(absolutely essential, again), and work done,which can be found out easily by using the First Law equation. By the way, PV diagrams are indeed necessary for calculating efficiency.