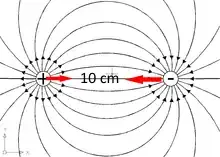
Imagine there is a proton confined in a box and we put an electron at 10 cm distance:
It gets an acceleration of thousands of meters/second^2 along a straight line joining the two CM's.
One would expect the electron to hit the positive particle in a fraction of a second, and stick there glued by a huge force, but this does not happen, even if we shoot the electron providing extra KE and velocity/momentum.
Is there a plausible explanation for that? Why doesn't the electron follow the straight force line that leads to the proton?
Edit
my question has been misunderstood: is not about orbitals or collisions. If it has an answer/explanation it is irrelevant if it refers to classical or QM physics. No explanation has been presented.
- We know that a) two protons can stick together even though repel each other via Coulomb force, it is legitimate then, a fortiori, to suppose that b) two particles that do not repel each other can comfortably sit side by side, almost touching each other:
- we also know that in a TV tube electrons leave the guns and hit the screen following incredibly precise trajectories producing pictures in spite of HUP and the fact the are a
"... a point particle having no size or position"
Now the situation I envisaged is very simple, and probably can be adequately answered step by step with yes/no or (approximate) figures:
- 0) When the electron is in the gun/box is it a point-mass/charge or is it a probability wave smeared over a region. when it hits the screen doess it have a definite size/position?
- 1) does electrostatics and Coulomb law apply here? do we know with tolerable precision what acceleration the electron will get when it is released and what KE and velocity it will aquire whenit ges near the proton?
- 2) if we repeat the experiment billion of times can those figures change?
- 3) according to electrostatics the electron should follow the force line of the electric field leatding to the CM of the proton and, when it gets there, remain as near as possible glued by an incredibly huge Coulomb force (picture 2 b). This does not happen,....never, not even by a remote probability chance. What happens, what prevents this from happening? Physics says that only a very strong force can alter the outcome of other laws.An answers states that QM has solved this long-standing mystery but does not give the solution.