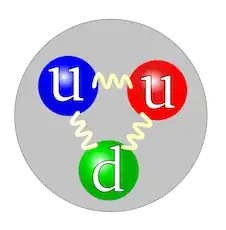
Consider say the proton. It is seems to be universally stated that a proton is "composed of three quarks, 2 up and a down, and you must have the three different 'colors'".
So, you can have blue-up, red-up and green-down, or maybe green-up, red-up and blue-down, and so on. You need only glance at the wikipedia page for an example illustration of this.
Something I don't understand is, imagine "some particular" proton (say inside one particular hydrogen atom). Is is the case that
(A) .. that particular proton is made of some combination. (Say, red-up, green-up, blue-down.) And then for all time, or for a long time, that particular proton is indeed made of that combination.
or, is it the case that
(B) .. that particular proton is continuously changing between the various possible combinations.
Which is it?
I generally understand the idea of color change - how gluons mediate the change of color between two quarks. This is explained widely for the general reader example, example.
However as I ask above, it is to me completely unclear whether that happens only occasionally (when?) or whether it is something that happens continuously (if so - exactly how often? 10 times a second? More, less?)
Furthermore. I was enjoying this excellent answer by John Rennie. The trouble is, that answer essentially says that the idea of a proton "being composed of three quarks" is in fact nonsense. Note too that this answer by another expert literally says ""red, blue or green" quarks do not exist".
What's the deal with this? For example, in particle accelerators, does one ever say "oh look ..... that proton there seemed to be a red-up, blue-up and green-down style of proton..". Or, in fact is this image:
... largely nonsense in terms of literally "what is a proton 'made' of"? Is it quite simply "utterly wrong" in any meaningful sense??
Again, even within that descriptive mode, which of "A" versus "B:" above is true??