Does an electron in p orbital move around nucleus or move randomly in any individual lobe of p orbital. if it were to move around nucleus then does p orbital move along with it?
3 Answers
As usual, you can re-express the wave-function in momentum space (it's just a Fourier transform away from the spacial wave-function for bound state which is really nice). But that does not tell you how the electron moves anymore than the spacial wave function tells you where it is. Instead, it tells you the probability distribution function for results of measuring the electron's momentum just as the position wave-function gives you the PDF for position measurements.
I'm not an expert in those measurements, but I've found a reference in which the momentum-state of electrons in xenon atoms was measured so sufficient precision to show clear relativistic effects (PDF link) (J.P.D.Cook, J.Mitroy, and E.Weigold, PRL. vol 52. no 13. p1116 1984).
The equivalent measurement for protons in a nucleus is done reasonably often---indeed my dissertation work included extracting the momentum-space PDF's for protons in a few nuclei.
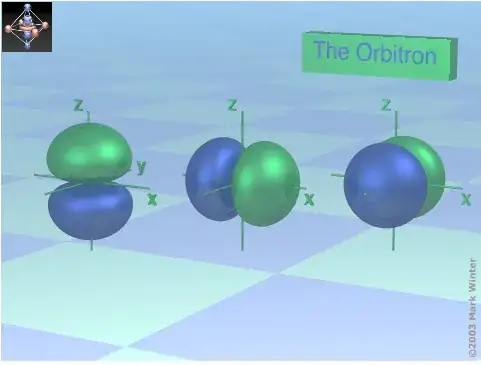
In addition to these two excellent answers I'd like to point out that the deceptively smooth surfaces of orbital graphical representations found in text books and web pages, like this excellent rendition of a 2p orbital, below are surfaces where the electron probability density is the same. In no way do they represent paths or 'orbits'.
- 35,561
The lobes are regions where you have a non-zero probability of finding an electron. These regions have varying shapes for different types of orbitals. It wouldn't make sense to think that orbitals are actually paths of some sort, where electrons whizz around. You'll know this is wrong if you try to understand the uncertainty principle.
The uncertainty principle prevents us from simultaneously measuring the momentum and position of an electron, up to sufficient accuracy.
Also, read: What is the uncertainty principle?
- 7,538