The following is a graph of the intensity of Bremsstrahlung generated by accelerating electrons to hit a target vs. its wavelength.
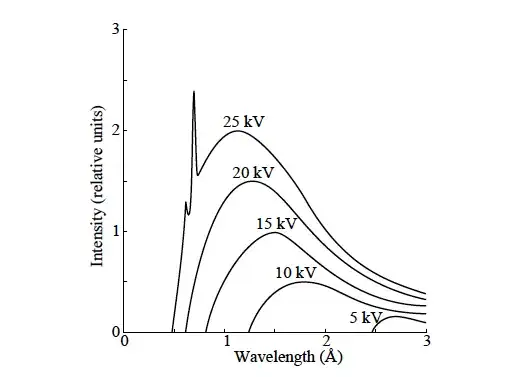
I'm wondering what causes the additional peaks for high energies? I have read in my physics script and on Wikipedia that "these peaks are characteristic of the material used as target", but what exactly is going on at the level of atoms?
Does the electron maybe go into some sort of bound state and thus loses - in addition to its kinetic energy - also some potential energy? Why would that only happen for high enough energies?