We know that electron(s) is(are) moving around the nucleus, that consists of protons and neutrons. But does nucleus itself has its own motion, its own momentum, or the nucleus is stationary? Now, is undisturbed atom stationary or is it "wandering" in space? If it does is that a spin or vibration or any type of an organized motion?
4 Answers
Electrons actually don't orbit the nucleus. This is a model that simplifies things. The most correct model of the atom that we have today is the Schrodinger model.
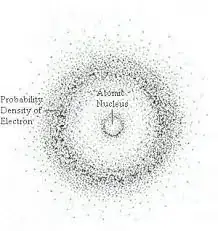
The denser the the cloud of dots, the more likely it is for an electron to be there. They do not have a predictable, regular motion. The nucleus also has this random positioning. It has a probability density.
- 4,019
Nuclei of course are subject to motion due to both thermal fluctuations and quantum fluctuations which give the nucleus some position/momentum uncertainty even in a vacuum. If you mean the protons and neutrons inside of a nucleus these definitely have motion as the nucleons are bound inside the nucleus into "orbitals" similar to electrons. They also occasionally bump into each other in the nucleus and scatter off of each other with large momentum.
- 2,845
As the simplest example of your question, consider the hydrogen atom, described in the atom's center of mass frame. In this frame, the electron has motion, in the usual sense in which things have motion in quantum mechanics. For example, it has a certain probability of having a momentum in a certain range of values. Since we're in the center of mass frame, and momentum is conserved, it follows that the proton's momentum is perfectly anticorrelated with the electron's. Therefore the proton does have motion. In momentum units, it has just as much momentum as the electron. However, in velocity units, it has much less.
In addition to this, more complex nuclei have internal motion. This follows from the Heisenberg uncertainty principle. For example, if a neutron is bound inside a lead nucleus, then its position uncertainty is no greater than the size of the nucleus, and therefore it has some momentum uncertainty.
if we assume electron and nucleon as a particle then according to concept of acceleration of centre of mass nucleus should also have some acceleration. let me explain how .... we well know that electron is moving around the nucleus in circular orbit and have some velocity which is changing its direction per unit time . This circular motion is due to centripetal accelration . We know that if net force on a body is zero then acceleration of centre of mass will also be zero . Since electron has centripetal acceleration but acceleration of centre of mass is zero nucleus must have some acceleration to counteract to vanish the acceleration of centre of mass.