Assume we have a balloon in a tank filled with a liquid , without any gravitational forces: We just know the Temperature, $V_0$ and $V_1$
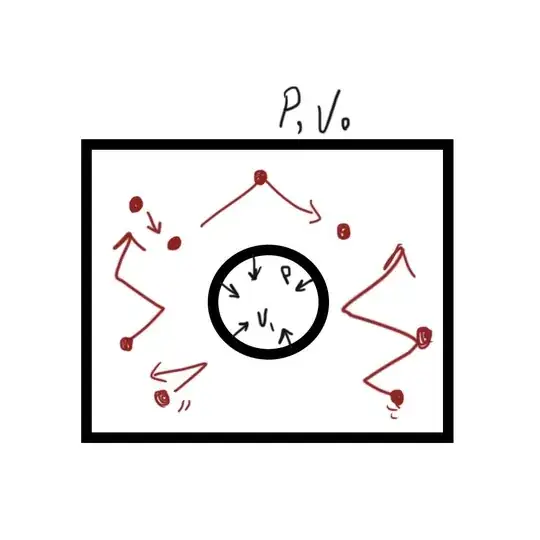
I want to calculate the pressure exerted from the liquid on the balloon. If it was just filled with gas I could easily calculate with $PV=nRT$, but this does not work for fluids.
Also by exclusion of gravity, the only source of pressure will be from the thermal energy ( fluid molecules moving and hitting on the balloon ) + internal cohesive energy.
UPDATE:
I think the only problem here is just finding the pressure exerted on the balloon by the liquid cohesive pressure ( in fact it is kind of equation of state problem ). I studied a lot since I asked this question. There are 2 pressures that I consider for using as P in this question:
1- Cohesive Energy density = 2.2973 GPa for water
2-Internal Cohesive Pressure= 168 MPa
My question is which one should be used here as $P$ ?