Your calculations are right, I think. I'm going to do the math here too and the pressure required is great. Your model, however, is wrong if you are imagining a piston compressing an ice cube. This is not a representation of what happens. Some points on the surface that touches the ice, in very small areas, provide the necessary pressure and the water formed is simply expelled from that place, giving way to more melting.
This problem, coincidentally, is literally a textbook case. Tester and Modell presents it as the problem 15-1:
The Clausius-Clapeyron equation describes the relationship between the pressure and temperature at the phase boundary between two phases of matter. Here's a step-by-step derivation of the Clausius-Clapeyron equation:
Step-by-Step Derivation
Consider a Phase Equilibrium:
Let's consider a system in equilibrium between two phases: phase 1 (solid) and phase 2 (liquid). At equilibrium, the chemical potentials of the two phases are equal:
$$
\mu_1(T, P) = \mu_2(T, P)
$$
where $ \mu_1 $ and $ \mu_2 $ are the chemical potentials of the solid and liquid phases, respectively.
Differential Change at Equilibrium:
For small changes in temperature $ dT $ and pressure $ dP $, the differentials of the chemical potentials are:
$$
d\mu_1 = \left( \frac{\partial \mu_1}{\partial T} \right)_P dT + \left( \frac{\partial \mu_1}{\partial P} \right)_T dP
$$
$$
d\mu_2 = \left( \frac{\partial \mu_2}{\partial T} \right)_P dT + \left( \frac{\partial \mu_2}{\partial P} \right)_T dP
$$
Since the chemical potentials must remain equal at equilibrium, their differentials must also be equal:
$$
d\mu_1 = d\mu_2
$$
Using Thermodynamic Identities:
The partial derivatives of the chemical potential with respect to temperature and pressure can be related to entropy and volume:
$$
\left( \frac{\partial \mu}{\partial T} \right)_P = -S_m
$$
$$
\left( \frac{\partial \mu}{\partial P} \right)_T = V_m
$$
where $ S_m $ is the molar entropy and $ V_m $ is the molar volume.
Applying the Identities:
Substitute these thermodynamic identities into the differentials:
$$
-S_{m1} dT + V_{m1} dP = -S_{m2} dT + V_{m2} dP
$$
Rearranging the Equation:
Collect terms involving $ dT $ on one side and terms involving $ dP $ on the other side:
$$
(V_{m2} - V_{m1}) dP = (S_{m2} - S_{m1}) dT
$$
Solving for $ \frac{dP}{dT} $:
Finally, divide both sides by $ dT $ and $ (V_{m2} - V_{m1}) $:
$$
\frac{dP}{dT} = \frac{S_{m2} - S_{m1}}{V_{m2} - V_{m1}}
$$
Relating Entropy Change to Latent Heat:
The change in molar entropy $ S_{m2} - S_{m1} $ can be related to the latent heat $ L $ of the phase transition:
$$
S_{m2} - S_{m1} = \frac{L}{T}
$$
where $ L $ is the latent heat per mole and $ T $ is the absolute temperature.
Substituting the Entropy Change:
Substitute this relation into the previous equation:
$$
\frac{dP}{dT} = \frac{L/T}{V_{m2} - V_{m1}}
$$
Final Form of the Clausius-Clapeyron Equation:
$$
\frac{dP}{dT} = \frac{L}{T \Delta V_m}
$$
where $ \Delta V_m = V_{m2} - V_{m1} $ is the change in molar volume during the phase transition.
The Clausius-Clapeyron equation is derived by considering the equilibrium condition between two phases, using the thermodynamic identities for the chemical potentials, and relating the changes in entropy to the latent heat of the transition. The final form of the equation is:
$$
\frac{dP}{dT} = \frac{L}{T \Delta V_m}
$$
This equation describes how the pressure of a phase boundary changes with temperature, taking into account the latent heat and the change in volume between the two phases.
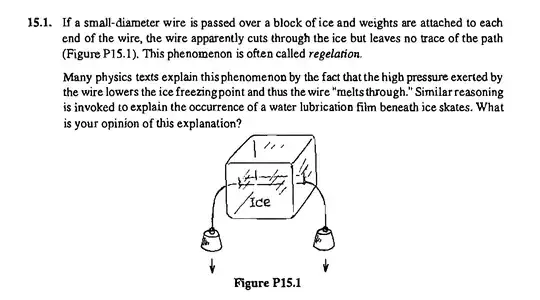
To solve Problem 15.1 from the Tester/Modell book, we need to understand the phenomenon of regelation, which involves a wire cutting through ice under the influence of attached weights without leaving a trace. This problem is often explained by the pressure-melting point relationship of ice.
Explanation and Analysis
Regelation refers to the process where ice melts under pressure and refreezes when the pressure is reduced. This phenomenon can be explained using the principles of thermodynamics, particularly the Clausius-Clapeyron relation, which describes the phase boundary between ice and water.
Clausius-Clapeyron Relation
The Clausius-Clapeyron equation is given by:
$$ \frac{dP}{dT} = \frac{L}{T \Delta V} $$
Where:
- $ \frac{dP}{dT} $ is the slope of the phase boundary in the pressure-temperature ( $ P $- $ T $) diagram.
- $ L $ is the latent heat of fusion.
- $ T $ is the absolute temperature.
- $ \Delta V $ is the change in volume during the phase transition.
For water and ice:
- The volume of water decreases when it freezes (ice has a larger volume than water at 0°C).
- Therefore, applying pressure lowers the melting point of ice.
Explanation of the Phenomenon
Pressure Application:
- When a wire with weights is placed over the ice, it exerts a high pressure on the ice directly beneath the wire.
- According to the Clausius-Clapeyron relation, this high pressure lowers the melting point of the ice.
Melting and Cutting:
- The ice under the wire melts because the pressure has lowered its melting point below the current temperature.
- The wire sinks through the melted water, continuously melting the ice beneath it as the pressure is maintained.
Refreezing:
- Once the wire has passed through, the pressure is removed, and the water refreezes back to ice because the pressure is no longer lowering the melting point.
- This explains why the wire can cut through the ice without leaving a trace.
Comparison to Ice Skating
Similar reasoning applies to the formation of a thin water layer beneath ice skates:
- The pressure exerted by the thin blade of the skate lowers the melting point of the ice.
- A thin layer of water forms under the skate, reducing friction and allowing smooth gliding.
Conclusion
The explanation provided in many physics texts, which attributes regelation to the high pressure exerted by the wire lowering the ice's freezing point, is consistent with thermodynamic principles. This reasoning also applies to the phenomenon observed in ice skating.
The wire cuts through the ice due to the high pressure it exerts, which lowers the melting point of the ice directly beneath it. The ice melts under the wire, allowing the wire to pass through. Once the wire has passed, the pressure is released, and the water refreezes, leaving no trace of the wire's path.
This phenomenon is well-explained by the Clausius-Clapeyron relation and is an excellent demonstration of the interplay between pressure, temperature, and phase changes in thermodynamics.
To calculate the weight necessary on each side of the nylon line to cut through a cube of ice, we need to determine the pressure required to lower the melting point of ice below the ambient temperature. Here are the steps to approach this problem:
Determine the Required Pressure to Lower the Melting Point:
Use the Clausius-Clapeyron equation to find the relationship between pressure and melting point.
Calculate the Force Required:
Convert the pressure into the force exerted by the nylon line.
Convert Force into Weight:
Calculate the mass (weight) required to exert this force.
Step 1: Determine the Required Pressure
First, we'll use the Clausius-Clapeyron equation to find the pressure required to lower the melting point of ice to below 0°C.
The Clausius-Clapeyron equation is:
$$ \frac{dP}{dT} = \frac{L}{T \Delta V} $$
Where:
- $ L $ is the latent heat of fusion of ice, $ 334 \, \text{kJ/kg} = 334,000 \, \text{J/kg} $
- $ T $ is the melting temperature of ice in Kelvin, $ 273 \, \text{K} $
- $ \Delta V $ is the change in volume per unit mass when ice melts, $ \Delta V = V_{\text{liquid}} - V_{\text{solid}} $
The density of ice is $ \rho_{\text{ice}} = 0.917 \, \text{g/cm}^3 = 917 \, \text{kg/m}^3 $, and the density of water is $ \rho_{\text{water}} = 1 \, \text{g/cm}^3 = 1000 \, \text{kg/m}^3 $.
$$ \Delta V = \frac{1}{\rho_{\text{ice}}} - \frac{1}{\rho_{\text{water}}} = \frac{1}{917} - \frac{1}{1000} \, \text{m}^3/\text{kg} $$
$$ \Delta V = 0.0010904 - 0.001 = 0.0000904 \, \text{m}^3/\text{kg} $$
Now, plug these values into the Clausius-Clapeyron equation:
$$ \frac{dP}{dT} = \frac{334,000 \, \text{J/kg}}{273 \, \text{K} \times 0.0000904 \, \text{m}^3/\text{kg}} $$
$$ \frac{dP}{dT} = \frac{334,000}{24.6912} $$
$$ \frac{dP}{dT} \approx 13,529 \, \text{Pa/K} $$
To lower the melting point by 1°C (1 K), the pressure required is approximately 13,529 Pa.
Step 2: Calculate the Force Required
Assuming we need to lower the melting point by approximately 1°C (enough to initiate melting under the line), the pressure required is:
$$ P = 13,529 \, \text{Pa} $$
The area of contact of the nylon line with the ice depends on the diameter of the line. Let's assume a standard nylon fishing line with a diameter of 0.5 mm (0.0005 m).
The contact area $ A $ of the line with the ice is the width times the length of the side of the cube:
$$ A = \text{diameter} \times \text{length} $$
$$ A = 0.0005 \, \text{m} \times 0.3 \, \text{m} = 0.00015 \, \text{m}^2 $$
The force $ F $ required is given by:
$$ F = P \times A $$
$$ F = 13,529 \, \text{Pa} \times 0.00015 \, \text{m}^2 $$
$$ F \approx 2.029 \, \text{N} $$
Step 3: Convert Force into Weight
To convert this force into the equivalent weight, we use the relation:
$$ F = m g $$
Where $ g \approx 9.81 \, \text{m/s}^2 $.
$$ m = \frac{F}{g} = \frac{2.029 \, \text{N}}{9.81 \, \text{m/s}^2} $$
$$ m \approx 0.207 \, \text{kg} $$
Therefore, the weight necessary on each side of the line to cut through a 30 cm cube of ice using a 0.5 mm diameter nylon line is approximately 0.207 kg.

