What does it mean to say that the conventional Raman effect is non-resonant? And, how/why does resonant Raman give a stronger signal than the non-resonant type?
1 Answers
The normal nonresonant Raman scattering happens when a photon interacts with a molecule; the molecule absorbs the photon momentarily and re-emits it with slightly less energy.
In an energy diagram, that looks like this.
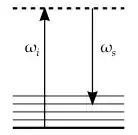
The frequency of the incoming photon is $\omega_i$, and the frequency of the scattered photon is $\omega_s$. The thick lined level is the ground state of the molecule, and the thin solid lined levels are vibrational states that are only slightly above the energy of the ground state.
The dotted line is a virtual energy state, which doesn't actually exist; but since the photon is re-emitted such a short time later, that's OK.
Most of the time, this doesn't happen; it's very uncommon compared to Rayleigh scattering, where $\omega_s=\omega_i$. That's why the signal from nonresonant Raman scattering is so low. Only a tiny, tiny fraction of scattered photons have their energy changed due to Raman scattering.
Now, resonant Raman scattering is exactly the same thing as nonresonant Raman scattering, except that $\omega_i$ is such that the virtual state's energy level is very close to one of the molecule's excited states (which are not virtual, but very real indeed.)
Being so close to an excited state makes the Raman process much more likely to occur. That's why the signal is so much stronger and can be used to detect much lower concentrations of a substance than conventional Raman can.
For more information, read the Wikipedia page.
- 4,354