I am replying to this because you seem to be a student, and not so clear on the statements.
I have read that during fission and fusion processes, there is some kind of equilibrium between the single nucleus and the disintegration products, so they are constantly being converted into each other.
" I have heard" is not enough, you should give a quote or a link. The statement is wrong. During fission a large nucleus breaks up because its component parts are more stable and the total energy balance is positive.
Look at this binding energy per nucleon in a nucleus curve:
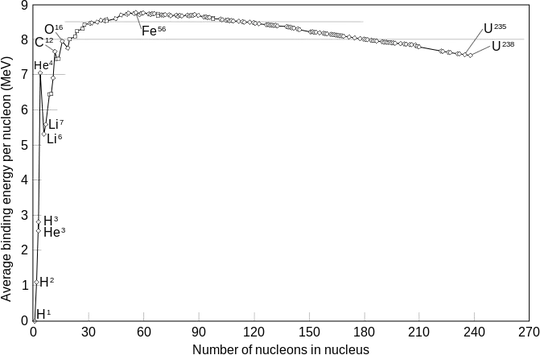
Below the top of the curve, putting together more nucleons gives energy, from the top of the curve to the right, removing a nucleon releases energy.
Furthermore, the energy after the fission of a single parent nucleus into two daughter nuclei is less than the energy required to fuse the two nuclei back together again.
This also is an out of context quote, you should give a link. According to the binding energy curve per nucleon it is a wrong statement
So if there is an equilibrium, how is the fusion energy achieved?
There is no equilibrium in laboratory conditions. Even in the center of the sun, more nuclei fuse than separate, otherwise the sun would non be the source of energy it is.
The fusion energy is released because two deuteron particles tied into one nucleus will have to release energy, as seen in the binding energy curve.
The fission energy happens because heavy nuclei are metastable in the sense that they could be pushed to break up into smaller parts more tightly bound releasing the binding energy of the large system.
Where did the extra energy come from?
The extra energy for fusion comes from the original existence of hydrogen helium atoms.
This happened during the Big Bang, according to the present model of creation of the universe. Atoms up to Fe in the binding energy curve were created in nucleosynthesis time. The heavier atoms were given energy by large explosions of heavy stars, like supernovae explosions, during the early universe days. All matter as we see it now was given its energy content at those early times , from the original impulse that generated the Big Bang.
