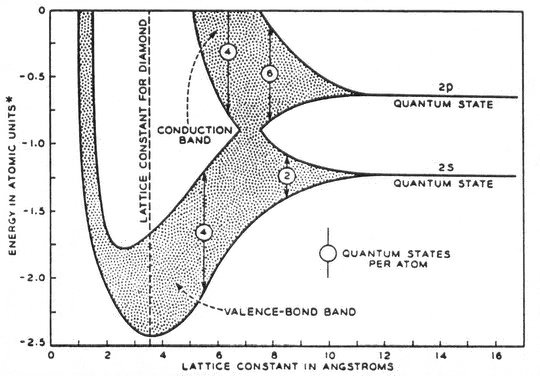
The maximum you can strain silicon, for example, before crystalline defects are introduced is ~1%, at which point the conductivity would go down. In the diamond case, the diagram indicates that you’d have to strain it by 200% (i.e. the atoms would have to be spaced twice as far apart as their equilibrium distance) to have no bandgap. This would be far too energetically unfavourable to happen. The material would break apart or be extremely defective well before this amount of strain is reached.
In other words (@JohnRennie), since this is a calculated band structure, there is no way to achieve this effect in diamond because it would fracture long before the C-C distance became large enough to merge the bands
Carbon can be very conductive (some forms of carbon nanotubes for example are metallic), but carbon in these forms is definitely not diamond.
 (source)
(source)