Why does iron sink in molten iron whereas ice floats on water?
Both are solid states of their own form, so why is one floating and the other sinking?
Why does iron sink in molten iron whereas ice floats on water?
Both are solid states of their own form, so why is one floating and the other sinking?
Solid water (ice) is one of the few known solids whose density is lower than that of its liquid form. Yes water is special! (but very much so in its chemical properties too) Due to the crystal structure of the solid phase of water, the molecules arrange themselves in a rigid, ordered fashion and end up being, on average, farther apart from each other (than they are in the liquid phase), and thus less dense. Less dense things float because of buoyancy. Thus, in most solids, such as iron, the molecules arrange themselves in a rigid, ordered fashion that ends up being, on average, closer from each other than they are in the liquid phase, and thus more dense. that one makes it closer and the other farther apart than liquid. Why water molecules separate and iron atoms get closer is more difficult to explain, to be able to show that, you have to actually calculate it using QM models. There is no intuitive argument, as far as I know, that will show you why one gets denser and the other less so.
Basically, it has to do with the density of the material as a function of temperature.
The density of iron increases as it cools, that is, solid iron is more 'packed tight' than when it is melted. This is understandable, since the kinetic energy of the iron atoms decreases as the temperature drops (ie: the average velocity of the atoms decreases), allowing them to pack more tightly in a given volume. This would be the 'expected' behaviour of most matter.
As you might expect, liquid water is generally similar, in that, as it is cooled, the kinetic energy of the molecules decreases and the density of the water increases, until we get down to $4^o C$, when something interesting starts to happen.
When the temperature drops below $4^o C$, the hydrogen bonds effectively 'hold' the water molecules further apart as a hexagonal crystal lattice starts to form.
The effect of the hydrogen bonding can be thought of as a 'relatively weak ionic' bond, in that at low temperatures the molecules begin to arrange themselves in a crystalline structure (the hexagonal crystal lattice). Basically, below $0^o C$ water becomes ice (an open hexagonal crystal) in which the water molecules are 'locked' in to an arrangement where there is more 'space' than the liquid state.
Ice floats because it is less dense as ice than it is as liquid. The water in ice expands by about 9%.
Density of water at $0^o C$ is 0.9998 g/mL
Density of ice at $0^o C$ is 0.9167 g/mL
That's why icebergs float with about 9% of their mass above the surface of the water.

Further cooling of ice, that is, below $0^o C$, results in an increase in density with respect to ice at $0^o C$, similar to solid metal.
So the 'anomalous' behaviour of water happens between $0$ and $4^o C$.
To learn more about molecular bonding (and the hydrogen bond in particular), refer to Chapter 12 of "The Nature of the Chemical Bond and Structure of Molecules and Crystals", by Linus Pauling.
Here's a quick summary.
Normally, hydrogen only forms one covalent bond, eg: the bonds between $H$ and $O$ within each $H_2O molecule.
However, when hydrogen forms such a bond with a highly electronegative atom (such as oxygen, nitrogen or fluorine), it leaves a slightly positive charge around the hydrogen atom. The presence of unbonded electrons in the outer shell of the oxygen atom give it a localised 'positive' charge, so that the water molecule is 'polar'. Adjacent molecules of water are thereby attracted to each, which is why water is a liquid at atmospheric conditions, something quite rate amongst molecules with similar atomic weight. It also gives water the ability to dissolve ionic solids.
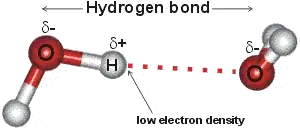
So, since iron does not have such hydrogen bonds, it behave more like 'regular' matter (with respect to its density variation as a function of temperature).
Water is a little different. Water's hydrogen bonds makes ice less dense than water. It basically pushes on the ice making it less dense. Iron is not like this. Molten iron is less dense and there is nothing to make it more dense. Iron is more solid and denser. All in all, water is of the few substances were its liquid form is denser than its solid form.