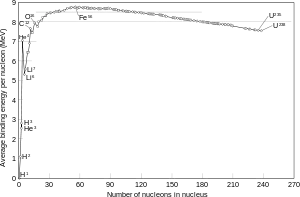
Given this nuclear reaction:
$^3_1\mathrm H\to {}^3_2\mathrm{He}+e^-+\bar{\nu}$
and knowing the binding energies:
$BE(^3_1\mathrm H)=8.48 \,\mathrm{MeV}$
$BE(^3_2\mathrm{He})=7.72 \,\mathrm{MeV}$
If I calculate the mass defect (obviously considering the binding energies in the mass calculation) I obtain a positive value:
$M(^3_1\mathrm H)c^2=2809.08 \,\mathrm{MeV} > M( ^3_2\mathrm{He})c^2+M(e^-)c^2=2808.991 \,\mathrm{MeV}$
as expected for a spontaneous decay. Considering the binding energies I have written above I expect the $^3\mathrm{H}$ to be more stable than $^3_2\mathrm{He}$.
My question is: why does this decay occur?