Current physics has established that the underlying framework is quantum. A basic principle of quantum mechanics is the Heisenberg Uncertainty Principle, HUP.
This ensures that for particles there can be no exact measurement or condition of 0 kinetic energy, so an ensemble of particles cannot have definite temperature of 0.
Photons are also particles and permeate the observable universe, even the emptiest of space, this is known as the left over radiation from the time that the universe became transparent to the electromagnetic waves . It is called cosmic microwave background, CMB, and its average energy is 2.7K.
This is what we observe:
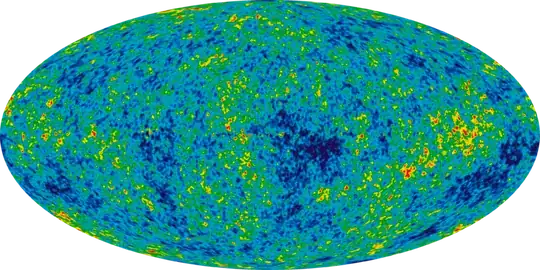
All-sky map of the CMB, created from 9 years of WMAP data
There is no zero, although there exists a cold spot:

The "cold spot" is approximately 70 µK colder than the average CMB temperature (approximately 2.7 K), whereas the root mean square of typical temperature variations is only 18 µK.
Thus, even if our universe is huge , there does not exist a spot without photons , and the HUP will ensure that there exists no zero K temperature.
Please also to remember that quantization of gravity will fill the space with gravitons, which will also be elementary particles and carry some energy, no matter how small, and will obey the HUP too. So in any interesting Universe there can be no 0K .

