Consider the following schematics of a Bainbridge mass spectrometer
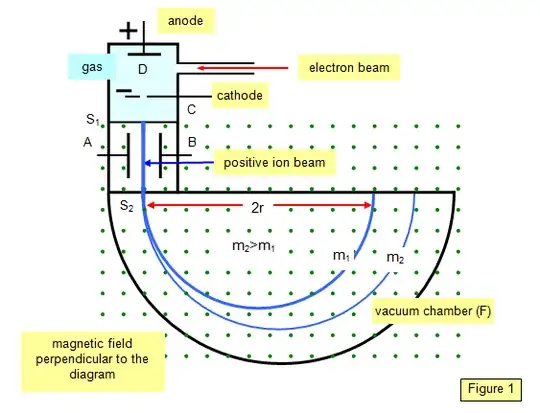
Suppose the gas contains two sorts of atoms (for example two isotopes of one element) with different masses. The electric force between the electrodes of in the gas chamber which accelerates them is $qE$, where $E$ is the electric field and $q$ the charge of the ions. For simplicity consider the case that you have one ion of sort A and one of type B each with equal charge q, but different masses $m_A$ and $m_B$ with $m_A > m_B$. Then the velocity of the $A$ before entering the Wien-filter will be smaller than hat of atom $B$ (because of it's mass). Furthermore suppose that the Wien filter only lets pass particles with velocity $v_A$, which is by assumtion the same as the velocity of our atom $A$. Because $B$ is faster than $A$, ions of type $A$ will pass the Wien-filter, but ions of type $B$ will not.
But then the magnetic field after the Wien-filter would be useless, because only ions of type $A$ would pass the filter.
So I guess that there must be another reason why the velocity distributions of type $A$ and $B$ atoms overlap after leaving the ion source such that both types $A$ and $B$ could reach the magnetic field after the Wien-filter.
Why is this the case? How can one quantitatively estimate how large the difference of $m_A$ and $m_B$ may be such that the velocity distributions of $A$ and $B$ overlap? Can you give me a quantitaive example of $A$ and $B$ and the concrete velocity distributions from experiments?
I am also looking for good references where those questions are discussed.