I'm revising for my exams at the moment and am seeing this notation everywhere
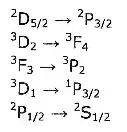
Can anyone tell me what these mean?
I'm revising for my exams at the moment and am seeing this notation everywhere
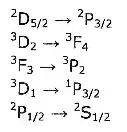
Can anyone tell me what these mean?
The expressions on either side of the equals sign are called term symbols. They specify the state of a system of electrons and they have the form $^{2S+1}L_J$. So for example $^{2}D_{5/2}$ corresponds to a system of electrons with $2S+1=2$, or in other words $S=1/2$; $L=2$; and $J=5/2$. They way I got $L=2$ was to use the correspondence $S \leftrightarrow 0,P \leftrightarrow 1,D \leftrightarrow 2,F \leftrightarrow 3,G \leftrightarrow 4, \cdots$
I believe that the arrows indicate that there has been a transition between two states.