A "normal, healthy" human eye has two types of light-sensitive cells in the retina: rods ("color blind", but capable of sensing low light levels) and cones: cells that are sensitive to different bands. See this figure for their relative sensitivity (from http://hyperphysics.phy-astr.gsu.edu/hbase/vision/colcon.html)
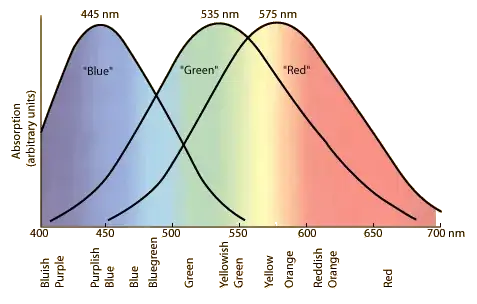
When you look at a spectrum of light, you can think of the eye's response as the result of the integral of $\int{I(\lambda)* sensitivity(\lambda)*d\lambda}$ for each of the three types of cones. It should be immediately obvious that different spectra (i.e. different "colors") can be perceived as being "the same" because of this way of processing the light. It gets further complicated because some people may be missing one or more types of cones ("color blind") so their perception of some colors may be different altogether.
The above is the reason that making "good white light" is hard. If you start with a bright (hot) incandescent source, you will be illuminating an object with every wavelength in the visible spectrum; but fluorescent lights (and LEDs) tend to create light in "bands". Thus, your perception of some colors will be different depending on the light source used. This is usually reflected in something called the "color rendering index" of the light source - where "100" represents perfect rendering, and lower numbers are indicative of a "lumpy" spectrum. The eye might still see this as "white" (because the source light excites the three types of cones evenly), but other "colors" may not show up very well since they do not in fact get illuminated.
An example of the different spectra is given at http://www.ledsmagazine.com/content/dam/leds/migrated/objects/features/10/2/11/Avnet_Fig2T_22513.jpg
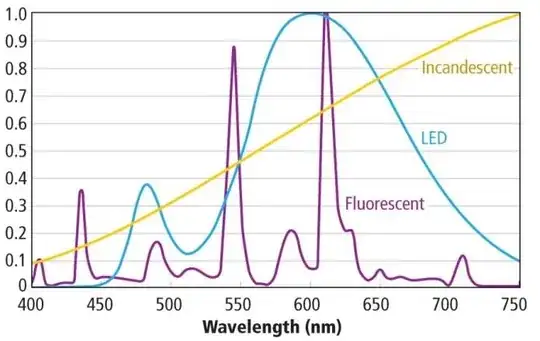
You can see that a typical incandescent lamp is biased towards red (because the filament is not as hot as the sun - this is why digital cameras do "white balance correction" and they usually have a "tungsten filament" setting). By contrast, a fluorescent lamp tends to have several distinct peaks in the emission spectrum - these are the emission peaks of the phosphors used to convert the UV light emitted in the gas of the tube into visible light. Finally, modern white LED technology is reaching a point where quite a wide range of excitation wavelengths are present, leading to higher color rendering fidelity.
Looking at the spectrum of the fluorescent tube above, if you had an object that was "black" except for a reflection in a narrow band at 570 nm, it would look black with such a source, when it ought to look orange. Yet if the same object was reflective at 545 nm (the emission peak), it would look bright green. An object that was a mix of "colors" in that narrow range would look very different in fluorescent light than in sunlight.
So the answer to your question is really - it's in the eye, not the brain.

