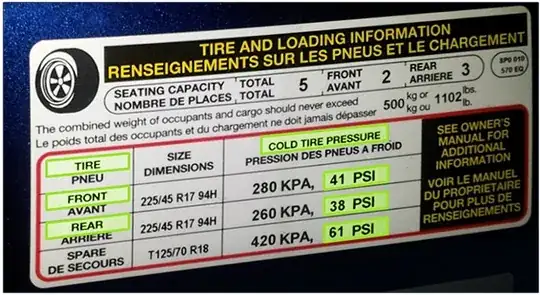
Correct Tire Pressures
As @Random832 mentioned in the comments, the information for my 2004 VW Passat B5 was not in the door jam of the drivers' side, but rather it was actually on the inside of my fuel filler lid. Here is what it looked like:

As you can see, the pressure here varies for the approximate amount of weight that the car is carrying, but also the pressure is only in bar. Given that 1 Bar = 14.5038 PSI (Pounds-Force per Square Inch) approximately, here are the manufacturer recommended tire pressures for my 2004 VW Passat B5:
+--------+-------------+------------+
| Weight | Front Tires | Rear Tires |
+--------+-------------+------------+
| Light | 30 | 28 |
+--------+-------------+------------+
| Heavy | 33 | 42 |
+--------+-------------+------------+
All values in the table are recorded in PSI and rounded to the nearest integer.
Sidewall Markings
As @Paulster2 mentioned, the PSI markings on the sidewalls were maximum values and should never be filled to this pressure for normal driving usage. The design of the car was not optimised for these higher tire pressures. It could also be dangerous if your tires are at the maximum and you drive over a large pothole of something similar.
Temperature
As the air temperature increases, the tiny air particles gain kinetic (movement) energy. They move faster at higher temperatures, which results in more successful particle collisions as long as volume remains constant (which it would be inside an automotive car tire). The energised air particles can not disperse at they are trapped (for the most part) inside the car tire and this causes an increase in pressure. In other words pressure is directly proportional to temperature (P α T).
As pressure increases by 10 degrees Celsius, the pressure will increase approximately 2-3 PSI. See this post (the approved answer) for a really nice graph and more details on the subject of car tire temperatures and the effect that has on tire pressures.