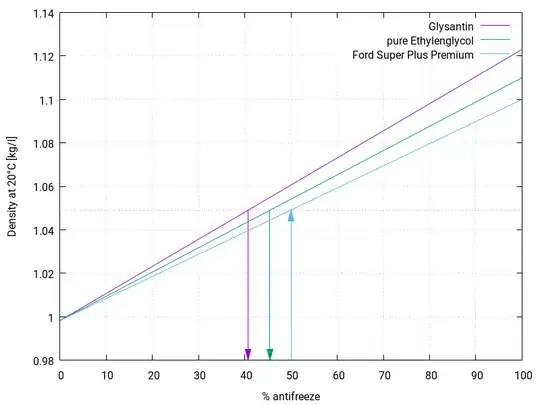
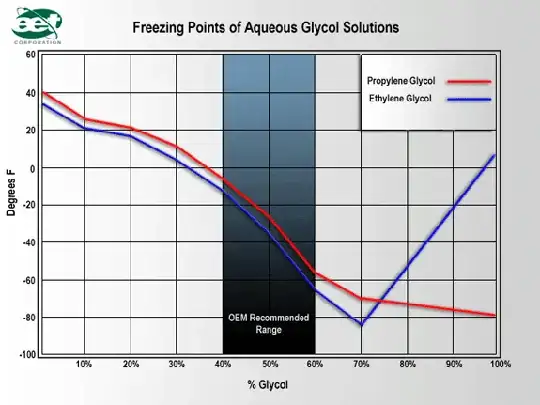
To keep things short I poured 200ml of concentrated genuine ford super plus premium antifreeze (Ethylene glycol based) within a bowl. Then added 200ml of water to dilute the solution to a 50/50 mixture. It states on the bottle when mixed 50/50 with water the antifreeze should protect to -37 degree's Celsius however when tested with a floating balls tester it shows that the solution would only protect up to -23 degrees. To investigate further I purchased two more hydrometer (different manufacturers) which tested the solution at -23 same as the first hydrometer. I don’t believe that three hydrometer from different manufacturers are going to lie to me so I’m puzzled.
After some research I found that -37 is the protection level that a 50/50 mixture should provide this is why I am confused.
My question is what should I do? Should I add more antifreeze increasing the strength to -37 or should I keep the solution at -23 when using within my car? If there is anyone that can give information to why this may be I would be more than grateful.
Thank you all for your time