Let me sum up your limitations of a CR2032:
- 10mA is about the max current you
want to pull from a single, it is
easy to put them in parallel, but a
large amount of testing(more than
2000 batteries worth) has confirmed
this.*
- They can be purchased to have
400mAh, the less current you pull
the closer to this it will be,
pulling more then 1mA decreases this a decent
bit.**
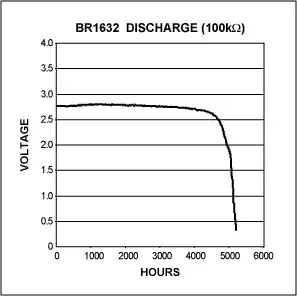
- Under a 1mA load they will
decay all the way to 1.5V before
they fail, they will be at 2.7V
almost right away.
- You can measure an almost full voltage on them with a multimeter when they are dead. This is solved by placing a load on them.***
- If you are lazy, it is very easy to tell how much charge they have left by how much they make your tongue tingle. Your tongue acts as the load and measures. This is probably by far the easiest way to test them, although it does pull a decent bit of current.
I think Thomas wrote a good answer, I just thought it might be helpful to give some details of the coin cells since it seems you have used AA quite a bit.
*Wikipedia says up to 15mA pulsed, but we confirmed that up to 1mA shows a nearly consistent capacity.
**Wikipedia shows a standard that is a bit lower, but my company would always purchase 400mAh or 450mAh CR2032. When you buy a "standard battery" you can expect 200mAh it seems.
*** People often will measure batteries without load, when someone tells you on a project that ran out of power early, ensure their original battery measurement was under load, very easy mistake.