I am reading an article (Khomenko, Electrochimica Acta 2005, 50, 2499-2506) that investigates the capacitance of carbon-based electrodes for use in supercapacitors. In particular, they use cyclic voltammetry (CV) to determine two types of electrodes (a PPy/MWNTs electrode, Figure 3, and a PANI/MWNTs electrode, Figure 4):
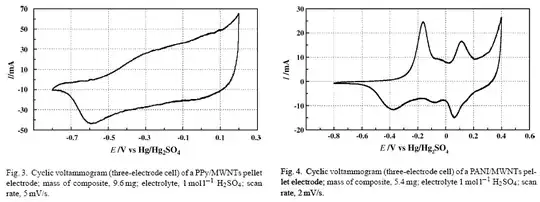
From these CV plots, the authors determine the capacitance values of each electrode:
The capacitance value was obtained from the CV curves according to the equation \$C = \frac{i}{s}\$ where \$i\$ is the average current and \$s\$ is the potential sweep rate. According to this formula, the cathodic charge determined by integration of the negative sweep of the CV curves is very high for such electrodes. The capacitance is \$670 \text{ F/g}\$ and \$506 \text{ F/g}\$ of electrode for the PANI and PPy composite electrodes, respectively.
I have a few questions about this method:
Why do the authors say that "the cathodic charge determined by integration of the negative sweep of the CV curves is very high"? Why do they refer to the cathodic charge, rather than the anodic charge?
What is meant by the average current \$i\$? What sort of average is meant? Do they mean consider a single sweep (either the part of the CV curve in which \$E\$ goes from negative to positive, or the part of the CV curve in which \$E\$ goes from positive to negative), and then integrate this sweep to find the average \$I\$? Recall from calculus that the average value of a function \$f(x)\$ in the interval \$a \leq x \leq b\$ is given by \$\bar{f} = \frac{1}{b-a} \int_a^b f(x) \; dx\$. Then does this mean that $$i = \frac{1}{\text{0.4 V } - \text{ -0.8 V}} \int_{\text{-0.8 V}}^{\text{0.4 V}} I(E) \; dE \;\;\;\;\text{ for the (-) to (+) voltage direction}$$ or $$i = \frac{1}{\text{ -0.8 V } - \text{ 0.4 V}} \int_{\text{0.4 V}}^{\text{-0.8 V}} I(E) \; dE \;\;\;\;\text{ for the (+) to (-) voltage direction}$$?
Hi Andrew, based on the answers you are receiving, it seems as though these questions aren't really dealing with chemistry concepts, and as such are off topic. If you do not agree with this decision, please start a Meta topic regarding this so the community can discuss it.– Jul 22 '12 at 13:47