Is it possible to make lightbulbs or any other electric light source without using high tech equipment? Is there something that could be created in small workshop?
-
3A very hot copper wire will emit some light. Connect one to a voltage source capable of providing few tens of amperes... And some of the first electric lights were based on an arc between two graphite rods. – Eugene Sh. Apr 11 '18 at 18:27
-
1There used to be instructions for making arc lights using pencil leads (which are actually graphite, not lead.) – JRE Apr 11 '18 at 18:35
-
1You can make a sphere or enclosure with mirrors plated all the area inside. Then in the morning open it and let light goes in and then close it. At night when you open the enclosure the light will come out. Intensity depends on how much you harvested in the morning. You can trap the photons by this way with a perfect mirror. Or use phosphorus element: https://en.wikipedia.org/wiki/Phosphorus for a weak intensity – GNZ Apr 11 '18 at 20:04
-
@JRE I doubt common pencil leads will work. It is actually much more glue than graphite, you probably rather make it burn with flame (the glue component) then arc. – Martin Apr 11 '18 at 20:07
-
@Martin: Nope. Seriously pencil lead. – JRE Apr 11 '18 at 20:28
-
1@GenzoWakabayashi You are not serious about capturing light between mirrors, are you? Also the OP is asking about electric light. – Eugene Sh. Apr 11 '18 at 21:08
-
@EugeneSh. I think he's late to 1st of April. – Harry Svensson Apr 11 '18 at 23:23
-
@Martin 2B pencil leads totally work. You only need a normal 2-3A bench power supply – Henry Crun Apr 12 '18 at 07:57
-
@GenzoWakabayashi this will never work, the mirror loss is too high. You need to use total internal reflection which is lossless. For example you could use optic fibre. The tricky part is closing the fibre loop fast enough, but a 300mm disc at 6000rpm travels 1mm fibre width in 10uS i.e 2000m of fibre. So you just need a 20km or so roll of fibre. – Henry Crun Apr 12 '18 at 08:04
-
@HenryCrun: Interesting, thanks for correcting me. I recall testing pencil leads long, long ago which ended up with igniting it to burn with (small) flame only, but no real arc. Maybe weak power supply or bad pencil or who knows, never tried it with pencils again since. – Martin Apr 12 '18 at 08:39
-
@martin The kids bake them out in the fire first to remove the binders. Also 2B is more carbon than clay, HB is more clay then carbon, and poor from memory. – Henry Crun Apr 12 '18 at 08:50
-
About "trapping" light: https://physics.stackexchange.com/questions/127262/can-light-be-trapped-theoretically – Eugene Sh. Apr 12 '18 at 13:29
4 Answers
Carbon arc lamp is probably the most low-tech kind of bright electric light. You need only carbon rods (welding equipment or extracted from common zinc battery cells for example), large resistor and somewhat powerful power source. See for example description here.
(Image from this site)
But be really careful if you try to reproduce this kind of light source yourself, it is quite dangerous for various reasons (fire danger, high voltage, production of strong UV light and noxious gases), see the warning in linked text too.
- 1,124
- 5
- 10
-
This looks like something doable. Do you know how powerful power source is required? Also how bright light can be? Is it comparable to lightbulbs? – Bobi Apr 14 '18 at 14:46
-
Carbon arc was used for anti aircraft searchlights in WW2, but you usually want two different sized carbons for best effect, and if you can get the copper coated ones normally used in old Cinema projectors you gain some useful efficiency. A few tens of volts at a few tens of amps is usually ballpark for a small one, going up to a few tens of volts at maybe 100A or so in a big unit. You will need to feed the carbons into the arc as they are slowly consumed, and watch the UV and reaction products, CO, CO2, NOx, Ozone, probably some buckyballs, none of it is exactly good for you. – Dan Mills Apr 16 '18 at 16:56
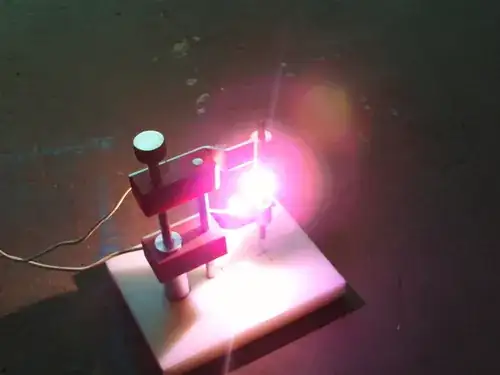
Yup Arc lights are the easiest by far. 2B pencil leads at a 2A bench power supply is quite adequate.
This was my kids at it. Actually at this point it has been turned into a spark transmitter.
- 5,363
- 11
- 12
Lime Light.
This is a ball of lime, heated with a high temperature gas flame.
Back in the day it was hydrogen and oxygen jets, directed onto the lime target. The light is quite excellent. We had great success with small cockle shells that had a bit of a bake out in the fireplace. Then heated with a MAPP gas flame. When the lime converts to CaO, and it hits temperature, it goes from dull orange, and bursts into white light. Stunning.
This is the same effect familiar to users of Coleman and Tilley mantle lanterns - which would probably be a more efficient way to use H-O
Making an electrolysis generator to run it off power would be standard stuff.
- 5,363
- 11
- 12
As long as your shop includes a vacuum pump, and you don't want long lifetime, sure.
Almost any conductor will give off light if you get it hot enough. Problem is, the hotter it gets the more efficient it is, and it's easy to melt a wire which is glowing white hot. Tungsten is what's used nowadays, and platinum will work pretty well, too.
Hot metal oxidizes quickly, so pumping out the air is a REAL good idea. If you don't have a vacuum pump, you can sort of work around it by simply replacing the air with argon, which you can get at any welding supply place.
Keep in mind that Edison got a patent for a bulb using carbonized bamboo for a filament, which would last about 1200 hours, in 1879, and tungsten first showed up commercially in about 1906. Not may folks think of 1879 as being "high tech", but YMMV.
- 60,521
- 2
- 37
- 97
-
1You might be able to burn the air out using a sacrificial "getter" filament. You first burn out the getter filament to absorb the oxygen, then fire up your light filament. Available getter materials are aluminium, magnesium and titanium wire. Titanium burns nitrogen, and its easy to get very fine Ti wire. Nichrome, stainless, Carbon, or Ti for filaments https://vacaero.com/information-resources/vac-aero-training/1166-getter-materials.html – Henry Crun Apr 12 '18 at 07:52