What are the experiments performed to determine the position of an electron inside the atom to verify the probability wave function data? Is it possible to do those experiments in real life?
3 Answers
Hyperfine interactions allow probing the wave function at the position of the nucleus, especially the contact density and the spin polarization of $s$ orbitals. It is a large field with several spectroscopic methods. The interaction influences both the electron energy and nuclear levels. The Mößbauer effect is especially striking. Using the Doppler effect, one can measure minute changes in the energy of nuclear transitions. The isotope Fe-57 (daughter of radioactive cobalt-57) has a very suitable transition at 14.4 keV. With the Doppler effect one can measure spectra with many lines that give information about oxidation state and magnetic moment of the atom.
The total shape of the orbitals can be measured by x-ray diffraction, which can be used to generate electron density maps of crystalline substances. Neutron diffraction can measure spin densities.
Photoemission spectroscopy is used to measure energy distribution functions which can be compared with theoretically computed densities of states.
With positron annihilation one can measure momentum distributions.
Clear examples of the spatial probability distributions come from X-ray crystallography. This technique is widely used for determining the atomic and molecular structure of crystals.
An incident X-ray beam is diffracted by a crystal into many specific directions. The X-ray diffraction pattern can be calculated back to the electron probability distribution.
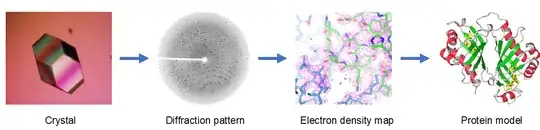
Picture taken from X-ray Crystallography Platform of www.creative-biostructure.com
- 42,352
Edit after change of title and content one hour after I answered the question, making a drastic change by inserting "inside an atom" in the title and the body
Original title to which the following is an answer:
What are the experiments performed to determine the position of an electron to verify the probability wave function data?
The clearest example of the probability distribution in space comes from the experiment: "electron of specific energy scattering off two slits of specific width and distance"
electron build up over time
One needs to do the experiment with the same boundary conditions for the electron accumulating events, the footprint of each electron on the screen. In the top frames it looks random, but slowly the interference seen demonstrates the wave function solution for the specific probability distribution.
A huge number of experiments accumulating probability distributions exist, the latest gave us the discovery of the Higgs.
- 236,935
